Original Articles: 2023 Vol: 15 Issue: 2
UV Spectroscopic and Iodometric Back Titration Analysis of Total Caffeine Content of Energy Drinks Available in Markets in Cross River State, South-South, Nigeria
Fredrick C Asogwa1*, Paul Ebo2, Richard S Ebugosi3, Immaculata, N Achara3, Gerald WUgodi4, Hope N Achieti-Atim1
1Department of Pure and Applied Chemistry, University of Calabar, Calabar, CRS, Nigeria
2Department of Microbiology, Tansian University, Umunya, Anambra, Nigeria
3Department of Biochemistry, Tansian University, Umunya, Anambra, Nigeria
4Department of Pharmaceutical Chemistry, Enugu State University of Science and Technology, Enugu, Nigeria
- Corresponding Author:
- Fredrick C Asogwa
Department of Pure and Applied Chemistry,
University of Calabar,
Calabar,
CRS,
Nigeria
Received: 25-Nov-2022, Manuscript No. JOCPR-22-81317; Editor assigned: 28-Nov-2022, JOCPR-22-81317 (PQ); Reviewed: 12-Dec-2022, QC No. JOCPR-22-81317; Revised: 21-Feb-2023, Manuscript No. JOCPR-22-81317 (R); Published: 28-Feb-2023
Abstract
Caffeine is an active ingredient of energy drinks that is often consumed to improve cognizance and physical cum mental alertness. This research aims to quantitatively analyze the caffeine content in serving volumes of energy drinks by spectrophotometric and iodometric back titration methods. In the spectrophotometric method, the determination of caffeine content was carried out using a maximum wavelength of 270 nm. The results show that the caffeine contents of the energy drinks were 37.40 mg/200 mL (Passion) <64.73 mg/250 mL (Bullet) <82.70 mg/355 mL (Power horse) <86.30 mg/400 mL (Predator) <114.68 mg/500 mL (Fearless) while the iodometric back titration method showed 57.50 mg/250 mL, 165.00 mg/500 mL, 160.00 mg/400 mL, 117.15 mg/355 mL and 40 mg/200 mL respectively for bullet, fearless, predator, power horse and passion. The labeled claims on the energy drinks were 78.75 mg/250 mL, 157.50 mg/500 mL, 120.00 mg/400 mL, 133.60 mg/355 mL and 50 mg/200 mL respectively for bullet, fearless, predator, power horse and passion which indicates that manufacturers reported higher values of caffeine content in their product possibly to make it attractive to consumers.
Keywords
Caffeine; Energy drink; Spectrophotometry; Iodometry; Back titration
Introduction
Caffeine is a bitter, white crystalline methyl xanthine alkaloid that occurs naturally in seeds, leaves or nuts of several plants and trees native to Africa, South America and East Asia and helps to protect them against herbivores and from competition by preventing the germination of nearby seeds, as well as encouraging consumption by select animals such as honey bees. Caffeine is structurally related to adenosine and acts primarily as an adenosine receptor antagonist with psychotropic and anti-inflammatory activities. Upon ingestion, caffeine binds to the adenosine receptors in the Central Nervous System (CNS), which inhibits adenosine binding. This inhibits the adenosine mediated down regulation of CNS activity; thus, stimulating the activity of the medullary, vagal, vasomotor and respiratory centers in the brain. The anti-inflammatory effects of caffeine are due to the nonselective competitive inhibition of Phosphodiesterases (PDEs).
Inhibition of PDEs raises the intracellular concentration of cyclic AMP (cAMP), activates protein kinase A and inhibits leukotriene synthesis, which leads to reduced inflammation and innate immunity. Some of the most common sources of caffeine include the beans (seeds) of two cultivated coffee plants, Coffee arabica and Coffee canephora (the quantity varies, but 1.3% is a typical value); the cocoa plant, Theobroma cacao, the leaves of the tea plant and kola nuts. Other sources include the leaves of yaupon holly, South American holly yerba mate and Amazonian maple Guayusa and seeds from Amazonian maple guarana berries [1].
Pure caffeine was isolated for the first time by a German chemist named Friedlieb Ferdinand Runge. He called it “Kaffebase” (i.e. a base found in coffee) at the instigation of Johann Wolfgang von Goethe. In 1895, the German chemist, Hermann Emil Fischer first synthesized caffeine from its chemical components. Caffeine (3,7-dihydro-1,3,7-trimethyl-1H-purine-2,6-dione) is also known as theine, methyl theobromine and 1,3,7-trimethylxanthine. Its molecular formula is C8H10N4O2·H2O and it consist of bicyclic molecules derived from the purine ring system. In its pure anhydrous form, caffeine is a bitter tasting, white, odorless powder with melting point of 235°C-238°C. Caffeine is only slightly soluble in water and alcohol, but it dissolves readily in chloroform. It is weakly basic (pKa of conjugate acid= ~0.6) requiring a strong acid to protonate it. While in solution, caffeine is essentially a neutral compound (pH=6.9) (Figure 1). An energy drink is a type of drink containing stimulants, usually caffeine, which is marketed for providing mental and physical alertness. They may or may not be carbonated, contain sugar, other sweeteners, herbal extracts, taurine and amino acids. Energy drinks belong to a class of products, in liquid form, advertised to enhance sports performance. The first energy drink appeared in the US in 1949 and was marketed as “Dr. Enuf”. In Europe, they were launched for the first time in 1987; then the market expanded across the world, becoming very popular especially after the launch of red bull in 1997. Since then, the energy drink market has grown dramatically, with various brands released worldwide. The annual consumption of energy drinks in 2013 exceeded 5.8 billion liters in around 160 countries. Currently, significant concerns have been raised about the safety of these products [2].
There have been several reports about adverse health effects associated with energy drinks. Despite this, manufacturers of energy drinks claim these products are suitable for consumers and that they are safe. In fact, the adverse health effects associated with energy drink remains controversial among scientists. There are limited comprehensive literature reviews that illustrate in detail the suitability and safety related to energy drink consumption, particularly among young adults. The aim of the present research was to determine the total caffeine content of energy drinks available in markets in cross river state, South-South, Nigeria and ascertain if the claimed caffeine levels on the labels of the energy drinks comply with regulatory standards [3-5].
MATERIALS AND METHODS
Materials
All chemicals and reagents used in this study were of analytical grade unless stated otherwise. Pure caffeine, dichloromethane and starch were purchased from Eyamson scientific ventures at no. 11 Eta Agbor road, Calabar, cross river state, Nigeria. Energy drinks were purchased directly from supermarkets and Watt market, Calabar, cross river state, Nigeria. Sodium thiosulphate, pure iodine crystals, sodium carbonate were obtained from the laboratory, department of pure and applied chemistry, university of Calabar, Calabar, Nigeria. All solutions were made with distilled water and stored at room temperature [6-8].
Apparatus and instruments
The apparatus used for the experiments include; beakers, measuring cylinders, electronic weighing balance, spatula, Erlenmeyer flasks, volumetric flasks, filter paper, burette, pipette, dropper, sample bottles, separating funnel and electric boiler. UV visible spectrophotometer was used for absorption measurement [9].
Preparations of solutions
Starch solution: 1.0 g of starch was poured into 10 ml of distilled water, stirred well and then transferred into 100 ml boiling water. The solution was stirred and boiled for one minute, then it was allowed to cool at room temperature and filtered [10].
Acid solutions: 1.0 M solution of HCl was prepared by measuring 8.3 mL of 37% HCl and pouring into a 100 mL volumetric flask containing small amount of distilled water and making up the solution to the mark with the distilled water.
Iodine solution: 0.634.4 g of iodine crystal was dissolved in 1000 cm3 distilled water to produce a 0.01 M solution of iodine.
Thiosulphate solution: 3.16 g of Na2S2O3 was dissolved in 1000 cm3 distilled water to get a 0.02 M thiosulphatesolution. The water used here was boiled and cooled before use.
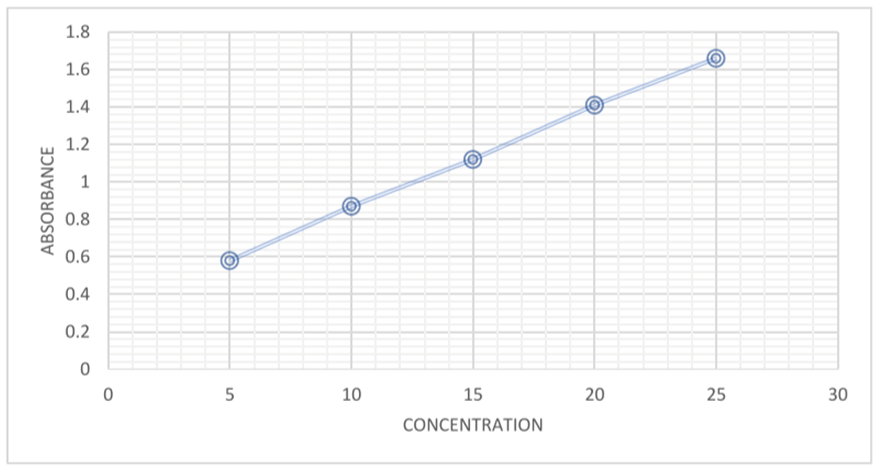
Preparation of caffeine standard solution: A 100 ppm stock standard solution of caffeine was prepared by weighing and dissolving 10 g of pure caffeine powder in 100 ml purified dichloromethane in a volumetric flask. Working solutions were then prepared by pipetting 5 mL, 10 mL, 15, 20 and 25 mL respectively of stock solution into separate 100 ml volumetric flasks and making it up to mark with dichloromethane to give concentrations of 5 ml/L, 10 ml/L, 15 ml/L, 20 ml/L and 25 ml/L respectively. The absorbance of these solutions was measured at 270 nm using UV visible spectrophotometer with 10 mm cuvette [11-13].
Determination of caffeine by iodometric back titration
Blank titration: 25 ml of the 0.01 M iodine solution was measured and poured into a 100 mL Erlenmeyer flask, 2.5 ml of 1.0 M HCl solution was added to the iodine solution and it was immediately titrated with the 0.02 M thiosulphate solution until the yellow color was almost gone (pale yellow). A few drops of starch indicator (three drops) were added and titration was continued until the blue color of the solution was gone. The procedure was repeated three times and the results recorded [14].
Determination of caffeine levels in the energy drinks: The energy drinks were used as supplied by manufacturers. 25 ml solution of 0.01 M iodine solution was measured and poured into a 100 ml Erlenmeyer flask, 2.5 ml of 1.0 M HCl solution was added to it and 25 ml of each brand of energy drink was also added to the mixture. The excess iodine solution was then titrated against a 0.02 M thiosulphate solution until the yellow color of the iodine was almost gone. A few drops of starch indicator were added and titration was continued until the blue color was gone. The results were recorded and the process repeated thrice for each sample of energy drink. The difference between the blank and each of the titration in a sample was used to determine the caffeine content found in each titration and the mean and standard deviation for the values for each sample was calculated to give the caffeine levels [15-17].
Caffeine extraction procedure for UV analysis
25 ml sample of the energy drink was drawn into a 25 cm3 pipette and placed into a 125 ml separation funnel, followed by the addition of 1 cm3 of 20% aqueous Disodium Carbonate (IV) (Na2CO3) solution and 20 cm3 analytical gradedichloromethane. The caffeine was extracted by inverting the funnel at least three times, venting the funnel after each inversion. The non-aqueous dichloromethane layer was removed into a clean 50 cm3 volumetric flask and another 20 ml of dichloromethane was added to the aqueous solution in the funnel and it was inverted three times, venting after each inversion and the dichloromethane layer was removed and added to the one in the volumetric flask and the volume was made up to 50 cm3 with dichloromethane. The procedure was repeated for all brands of energy drinks. The absorbance of each resulting solution was measured using UV visible spectrophotometer at 270 nm using a 10 mm cuvette.
RESULTS AND DISCUSSION
This study used samples of energy drink containing caffeine as active ingredient which serves as a stimulant or recreational drink. These drinks are very popular in the community especially with youths and university students. It is often used as a cognitive stimulant and to relieve fatigue and improve alertness. Analysis of caffeine in this study was determined by spectrophotometric and iodometric back titration methods. Spectrophotometry is a quantitative measurement of the reflection or transmission properties of a material as a function of wavelength. This method has the advantage of using a short time, low cost, robustness and capability for high precision. The use of spectrophotometry is widely applied in the analysis of caffeine levels in drinks. The UV visible spectrophotometric results are given in Tables 3-6. Determination of caffeine levels in the energy drink samples was done by extrapolation from the standard caffeine calibration curve and applying Beer’s law in the determination of the molar absorptivity of caffeine and thereby calculating the concentration of caffeine in each energy drink using their absorbances. The caffeine content of the studied energy drinks in increasing order was PA<BU<PH<PD<FE and the values were 37.510 ppm, 64.730 ppm, 82.697 ppm, 86.304 ppm and 114.680 ppm respectively. Iodometric back titration is a simple and accurate experiment for determining the amount of caffeine in an aqueous solution. The method requires simple laboratory apparatus and common chemicals [18].
I2 +2Na2S2O3→2NaI+ Na2S4O6 (1)
C8H10N4O2+2I2+HCl→C8H10N4O2. HI.I3+Cl (2)
Equation of reaction to determine caffeine level
The concentration of caffeine (i.e. caffeine level) can be calculated by following the reaction equations (1) and (2) and calculating the moles of iodine added and the moles reacted with the thiosulphate, the number of moles reacted with caffeine can be determined. But in this research, the caffeine levels in the energy drink samples were determined by calculating the volume difference between the blank titration and the average volume of each titration and hence, calculating the concentration by equivalence [19,20].
Iodometric back titration results
The results of caffeine determination in each sample of energy drink by iodometric back titration are presented in Tables 1 and 2.
| Energy drink | Caffeine content in mg/mL | Caffeine content in g/mL |
|---|---|---|
| BU | 0.23 ± 0.35 | 0.00023 ± 0.01 |
| FE | 0.33 ± 0.21 | 0.00033 ± 0.01 |
| PD | 0.40 ± 0.26 | 0.00040 ± 0.01 |
| PH | 0.33 ± 0.30 | 0.00033 ± 0.01 |
| PA | 0.20 ± 0.17 | 0.00020 ± 0.02 |
Table 1: Caffeine content in energy drinks from iodometric back titration in Mean ± SD.
| Energy drink | Labeled claim (mg/100 mL) |
Amount found (mg/100 mL) |
Labeled claim (mg/container) |
Amount found (mg/container) |
|---|---|---|---|---|
| BU (250 mL) | 31.50 | 23.00 | 78.75 | 57.50 |
| FE (500 mL) | 31.50 | 33.00 | 157.50 | 165.00 |
| PD (400 mL) | 30.00 | 40.00 | 120.00 | 160.00 |
| PH (355 mL) | 32.00 | 33.00 | 113.60 | 117.15 |
| PA (200 mL) | - | 20.00 | 50.00 | 40.00 |
Table 2: Comparison of the labeled claim and amount of caffeine found in energy drinks using iodometric back titration.
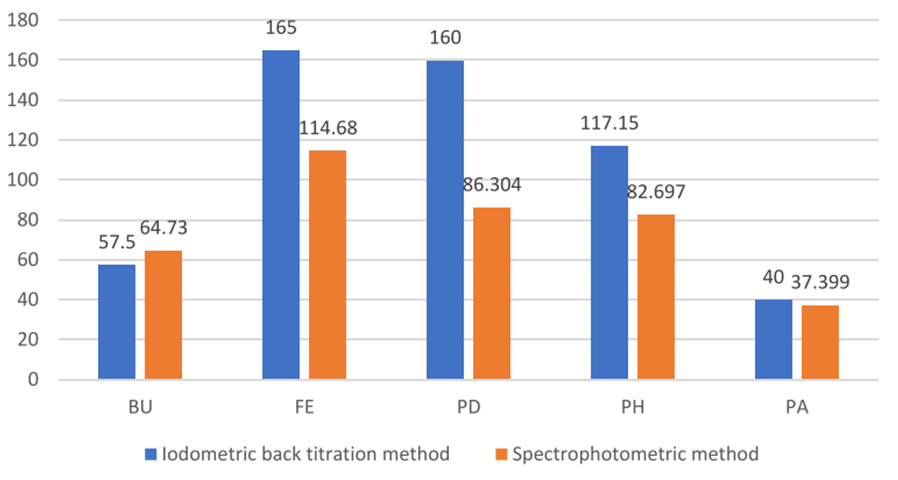
The caffeine levels in drink samples assayed were in the range of 40 mg-165 mg of caffeine per serving volume. The study showed that the estimated caffeine levels for Bullet (BH) drink was 57.50 mg/serving can, Fearless (FE) was 165.00 mg/serving bottle, Predator (PD) was 160.00 mg/serving bottle, Power Horse (PH) was 117.15 mg/serving can and that of Passion (PA) drink was 40.00 mg/sachet dissolved in 200 mL of water. The presence of other compounds in the energy drinks studied can be an interference resulting in the addition reaction with caffeine and also in iodometric back titration, the properties of iodine solution show that it is volatile (Figure 2). Therefore, some iodine can be lost during the experimental process, thus, contributing to titration errors.
Comparison of the results from the two methods show significant differences in caffeine levels in fearless and predator energy drinks, but the results of the other drinks were in close range (Figure 3).
All iodometric back titration results were higher than the spectrophotometric results except for sample BU where the latter was slightly higher than the former [21-23]. Both methods produced acceptable results of estimated caffeine levels as both values are well within the same range and also in the range of the stated labels of caffeine contents in the energy drinks. From the Iodometric back titration method, comparison of labeled claim and amount of caffeine in mg found in the energy drinks as seen in table, show that BU drink had 57.50 mg of caffeine against the labeled 78.75 mg, FE had 165.00 mg against 157.50 mg, PD had 160.00 mg against 120 mg, PH had 117.15 mg against the 113.60 mg and PA had 40.00 mg against 50 mg. While comparison of the results of spectrophotometric method with the labeled claims of the brands generally showed lower concentrations of caffeine as seen in Table 6 with BU having 64.73 mg against the labeled claim of 78.75 mg, FE had 114.68 mg against the labeled 157.50 mg, PD had 86.30 mg against 120.00 mg, pH had 82.70 mg against 113.60 mg and PA had 37.40 mg against the labeled claim of 50 mg. Although the spectrophotometric method generally gives more accurate results than the iodometric back titration method due to the smaller errors associated with instrumental methods of analysis, the results of titrimetric method gave caffeine levels closer to the labeled claims on the energy drink compared to those of the spectrophotometric method. The caffeine content in energy drinks were determined via iodometric back titration using a solution of pure iodine crystals in acidic medium to react with caffeine in the energy drink samples, with the remaining excess amount of iodine reacting with sodium thiosulphate, using starch solution as an indicator for the titration and also via UV visible spectrophotometry were a standard calibration curve was made from the absorbance of certain concentrations of caffeine standard solution in a UV visible spectrophotometer using dichloromethane as solvent [24,25]. Caffeine content in samples was calculated by extrapolation from the curve and applying Beer’s law to find the molar absorptivity of pure caffeine. The absorbance of the samples in the UV visible spectrophotometer was used to determine the concentration of caffeine in the samples (Table 3-6).
| Concentration (mg/L) | Absorbance |
|---|---|
| 5 | 0.58 |
| 10 | 0.87 |
| 15 | 1.12 |
| 20 | 1.41 |
| 25 | 1.66 |
Table 3: Absorbance of the resulting solutions of caffeine standard solution dilutions.
| Sample | Absorbance |
|---|---|
| BU | 1.8 |
| FE | 1.6 |
| PD | 1.5 |
| PH | 1.62 |
| PA | 1.3 |
Table 4: Energy drinks sample absorption in UV spectrophotometer.
| Energy drink samples | Conc. in mg/L | Conc. in mg/L*DF |
|---|---|---|
| BU (400 mL) | 6.473 | 12.946 |
| FE (500 mL) | 5.734 | 11.468 |
| PD (400 mL) | 5.394 | 10.788 |
| PH (400 mL) | 5.828 | 11.656 |
| PA (200 mL) | 4.674 | 9.350 |
*DF=Dilution Factor
Table 5: Caffeine content in energy drinks from spectrophotometric method.
| Energy drink | Serving size (mL) | Labeled claim mg per container | Amount found ppm per container |
|---|---|---|---|
| BU | 250 | 78.75 | 64.730 |
| FE | 500 | 157.50 | 114.680 |
| PD | 400 | 120.00 | 86.304 |
| PH | 350 | 113.60 | 82.697 |
| PA | 200 | 50.00 | 37.399 |
Table 6: Comparison of the labeled claim and amount of caffeine found in energy drinks using UV-visible spectrophotometry.
CONCLUSION
Determination of caffeine content in energy drinks by spectrophotometric and iodometric back titration methods yielded results with high accuracy. While the spectrophotometric method is more accurate due to the lesser errors associated with the method, the iodometric back titration method is still a very good method to use, especially as energy drinks do not contain much components that can hinder the iodine addition reactions on caffeine. This method is cheaper, requires simple laboratory apparatus and common chemicals only and has a wider range of uses. It is necessary to advise that manufacturers of energy drinks label the caffeine content of their products correctly and design their marketing messages to properly address and educate costumers on consumption pattern and adverse effects of excess caffeine in the body.
ACKNOWLEDGMENTS
The authors are thankful to all the technical staff and laboratory assistants in the department of pure and applied chemistry, faculty of physical sciences, university of Calabar, Calabar, for their valuable assistance during the period of the experiments.
FUNDING
No external funding was received.
CONFLICT OF INTEREST
All authors unanimously declared no conflict of interest.
AVAILABILITY OF DATA AND MATERIAL
All data and materials are available within the manuscript.
AUTHOR’S CONTRIBUTION
The research project was conceptualized, designed and supervised by Fredrick CA. All experiments, results analysis along with manuscript draft was conducted by hope NA. Chioma CJ and Gerald WU carried out results analysis and manuscript writing.
References
- Arauz J, Moreno MG, Cortés?Reynosa P, et al. J Appl Toxicol. 2013;33(9):970-979.
[Crossref] [Google Scholar] [PubMed]
- Benjo AM, Pineda AM, Nascimento FO, et al. Circulation. 2012;125(11):1447-1448.
[Crossref] [Google Scholar] [PubMed]
- Cheng M, Hu Z, Lu X, et al. Can J Cardiol. 2014;30(4):448-454.
[Crossref] [Google Scholar] [PubMed]
- Cornelis MC, Monda KL, Yu K, et al. PLOS Genet. 2011;7(4):e1002033.
[Crossref] [Google Scholar] [PubMed]
- Cunha RA, Agostinho PM. J Alzheimers Dis. 2010;20(1):95-116.
[Crossref] [Google Scholar] [PubMed]
- Yazid EA. J Trop Pharma Chem. 2019;4(6):271-280.
- Einöther SJ, Giesbrecht T. Psychopharmacology (Berl). 2013;225(2):251-274.
[Crossref] [Google Scholar] [PubMed]
- El Yacoubi M, Ledent C, Parmentier M, et al. Psychopharmacology (Berl). 2000;148(2):153-163.
[Crossref] [Google Scholar] [PubMed]
- Eskelinen MH, Kivipelto M. J Alzheimers Dis. 2010;20(1):167-174.
[Crossref] [Google Scholar] [PubMed]
- Graham TE. Sports Med. 2001;31(11):785-807.
[Crossref] [Google Scholar] [PubMed]
- Greenberg JA, Dunbar CC, Schnoll R, et al. Am J Clin Nutr. 2007;85(2):392-398.
[Crossref] [Google Scholar] [PubMed]
- Hackett PH. High Alt Med Biol. 2010;11(1):13-17.
[Crossref] [Google Scholar] [PubMed]
- Higdon JV, Frei B. Crit Rev Food Sci Nutr. 2006;46(2):101-123.
[Crossref] [Google Scholar] [PubMed]
- Klatsky AL, Hasan AS, Armstrong MA, et al. Perm J. 2011;15(3):19-25.
[Crossref] [Google Scholar] [PubMed]
- Lopez-Garcia E, van Dam RM, Willett WC, et al. Circulation. 2006;113(17):2045-2053.
[Crossref] [Google Scholar] [PubMed]
- Michael WK, David NM, Bright SA, et al. Int J Nutr Metab. 2018;10(3):16-22.
- Mora-Rodriguez R, Pallares JG. Nutr Rev. 2014;72:108-120.
[Crossref] [Google Scholar] [PubMed]
- Namdar M, Schepis T, Koepfli P, et al. PLoS One. 2009;4(5):e5665.
[Crossref] [Google Scholar] [PubMed]
- Nawrot P, Jordan S, Eastwood J, et al. Food Addit Contam. 2003;20(1):1-30.
[Crossref] [Google Scholar] [PubMed]
- Palatini P, Ceolotto G, Ragazzo F, et al. J Hypertens. 2009;27(8):1594-1601.
[Crossref] [Google Scholar] [PubMed]
- Pelchovitz DJ, Goldberger JJ. Am J Med. 2011;124(4).84-289.
[Crossref] [Google Scholar] [PubMed]
- Postuma RB, Lang AE, Munhoz RP, et al. Neurology. 2012;79(7):651-658.
[Crossref] [Google Scholar] [PubMed]
- Reissig CJ, Strain EC, Griffiths RR. Drug Alcohol Depend. 2009;99(1-3):1-10.
[Crossref] [Google Scholar] [PubMed]
- Rhoads DE, Huggler AL, Rhoads LJ. Pharmacol Biochem Behav. 2011;99(1):81-86.
[Crossref] [Google Scholar] [PubMed]
- Rogers PJ, Heatherley SV, Mullings EL. Psychopharmacology (Berl). 2013;226(2):229-240.
[Crossref] [Google Scholar] [PubMed]