Original Articles: 2025 Vol: 17 Issue: 2
Use of Silver Nanoparticles for Adsorption-based Methylene Blue Dye Removal in an Aqueous Medium
Vishnu Kumar Khandelwal*
Department of Chemistry, JECRC University, Jaipur, India
- Corresponding Author:
- Vishnu Kumar Khandelwal
Department of Chemistr,
JECRC University,
Jaipur,
India
Received: 28-Apr-2024, Manuscript No. JOCPR-24-133419; Editor assigned: 01-May-2024, PreQC No. JOCPR-24-133419 (PQ); Reviewed: 15-May-2024, QC No. JOCPR-24-133419; Revised: 08-Jan-2025, Manuscript No. JOCPR-24-133419 (R); Published: 15-Jan-2025, DOI:10.37532/0975-7384.2025.17(2).234.
Citation: Khandelwal VK. 2025. Use of Silver Nanoparticles for Adsorption-based Methylene Blue Dye Removal in an Aqueous Medium. J. Chem Pharm. Res., 17:234.
Copyright: © 2025 Khandelwal VK. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Methylene blue at low doses is found to be effective in enhancing energy in the cells of human body. Thus, it has found its way as supplement alongside it’s usage in textiles as dye. It is reported to beingtoxic, carcinogenic and non-biodegradable. This work shows the way methylene blue dye was adsorbed from an aqueous solution using silver nanoparticles. The evaluation and estimation of silver nanoparticles under various phases of experimentation was carried out using UV-Visible spectroscopy, Fourier Transform Infra-Red spectroscopy, Zeta Potential and Particle Size Analyzer. The samples contained silver nanoparticles, as indicated by the absorbance peak observed at approximately 420 nm. The size distribution analysis reported that the samples with average particle size of 47 nm is in the nano range. The colloidal properties, dispersion efficacy and stability of the nanoparticles were evaluated using Zeta potential. A strong negative value was indicative of the silver nanoparticles being stable and suitable for use in additional experiments. The presence of active bonds that could be present in the sample that could possibly interact with the methylene blue dye to decolorize it was determined using FTIR. The adsorption was determined to be efficacious post six hours of incubation with adsorption of up to three milligrams per liter of methylene blue dye.
Keywords
Silver nanoparticles; Dye adsorption; Discoloration; Incubation; Negative zeta potential
INTRODUCTION
Utility of dyes, be it in textile industries, food processing as coloring agents or in textile, paint, leather, cosmetics and pharmaceutical industries, has been to enhance the aesthetics of their products. Industries mostly use synthetic dyes with azo compounds as the primary ingredient [1, 2]. Statistics reveal that roughly 1.6 million tons of various dyes are produced annually, with 10% to 15% of this amount being routinely released into the open as effluent waste [3]. Although dyes give aesthetic value to products, they also contribute to several ill effects on human health. Because of their mutagenic and teratogenic qualities, they can cause allergies, hyperactivity and even seem to act as precursor for cancer [4]. They are also dangerous to the environment because the effluent wastewater from the afore-mentioned industries, contains dyes that are partially or completely not used. Thus, when disposed improperly into open environment, these dangerous dyes from wastewater contaminate the environment by seeping through the soil and into ground water [5].
The ramifications mentioned above make it imperative to stop the release of untreated effluent waste into the environment. Certain conventional approaches adopted to remove dyes include oxidation by electrochemical method [6], ozonation [7], degradation by photocatalytic method [8], use of activated carbon for adsorption [9], physical separation by way of coagulation and flocculation [10], forward osmosis [11] and usage of membranes for nanofiltration [12].
Further, the above-mentioned techniques have been found to have inadequacies such as low surface area for exchange for dye removal and high cost involved in multiple levels of treatment involved in the process. A variety of adsorbents have been employed to adsorb dye onto their surface. Palm kernel oil [13], calcium activated biochar [14], paste made of fly ash, POFA, kaolin, metakaolin and dolomite based geopolymer materials [15], leaf-based bio adsorbent [16], refined wood pulp known as microcrystalline cellulose [17] and so on are examples of these adsorbents. They offer a surface area for the degradation of toxic dyes, but more work needs to be done to improve the surface area of adsorbents that are already in place or create new ones that have a higher surface area. Nanoparticles are more dependable because they have a larger surface area and can effectively adsorb dye on their surface and break it down. These days, nanoparticles are applied with remarkable efficiency in a variety of fields, including bio-sensing, environmental monitoring, healthcare, agriculture and even dye removal [18]. Rhodamine B and indigo carmine were photo catalytically degraded using zinc oxide nanoparticles in one study [19].
Iron oxide [20], stannic oxide, iron doped zirconia, starch coated magnetic nanoparticles, polymer core shell, cobalt, magnesium aluminate and starch coated magnetic nanoparticles are some other nanoparticles that have been successfully used for dye removal. It is necessary to evaluate silver nanoparticles much more extensively. Their intrinsic properties are to be investigated using a variety of analytical and biochemical techniques. As a result, the current study has concentrated on the production of silver nanoparticles and their potential uses in wastewater treatment to remove methylene blue dye.
MATERIALS AND METHODS
Chemicals and instrumentation
Methylene blue was purchased from Himedia and trisodium citrate and Silver nitrate (AgNO3) were utilized in the silver nanoparticles (AgNPs) synthesis. The particle size distribution was determined using zeta size distribution, Particle Size Analyzer (PSA) and UV-Visible spectroscopy (UV-Vis), which were obtained from the Central Instrumentation Facility of MNIT Jaipur, India. The source of the Fourier Transform Infrared spectroscopy was MNIT, Jaipur. Analytical grade chemicals were all used in this investigation. The experiment used only distilled water.
Synthesis of silver nanoparticles
AgNPs were chemically synthesized in order to remove dye. Methylene blue dye was decolorized using AgNPs. The produced nanoparticles were kept in storage at 4°C prior to usage and additional research.
Characterization of AgNPs
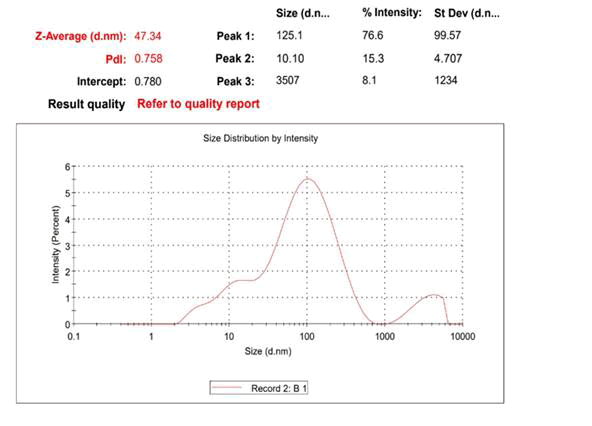
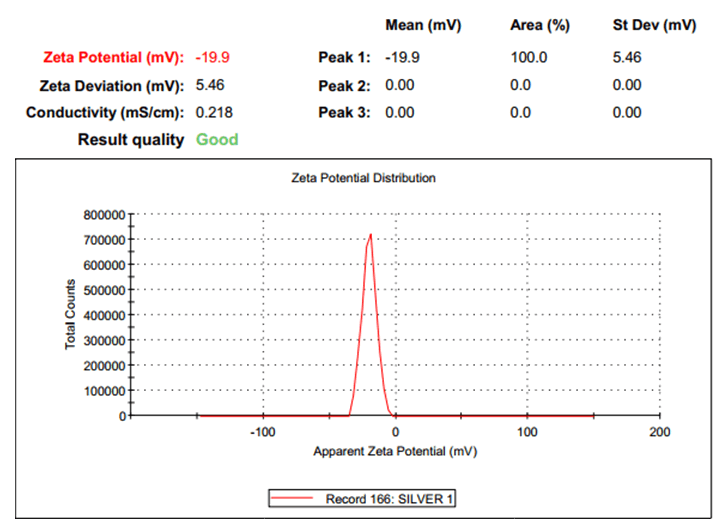
The absorbance of the sample was obtained across complete range of UV-Vis spectroscopy to determine optimum absorbance to characterize the AgNPs initially. The PSA will offer size distribution andindicates the percentage of particles of a certain size (or in a certain size interval) to determinethe mean size of the particles in the sample. At a standard temperature of 25ºC, the size distribution analysis was conducted using water as a dispersant and a system count rate of 449.5 kcps. The sample was then put through a Zeta sizer, which will reveal details regarding the particles conductivity at a negative potential. The zeta analysis was conducted under the same standard conditions as the size distribution analysis. The sample was put through TEM in order to confirm the precise size and shape of the particles that are being synthesized. The sample was subjected to FT IR to determine types of bonds present in the sample.
Dye removal using the AgNPs
AgNPs were used in a kinetic analysis of the discoloration of methylene blue dye following the successful confirmation of their synthesis. The amount of dye and nanoparticles present is vital to the process of adsorption. The first step involved measuring the methylene blue absorbance between 400 nm and 800 nm. A standard graph was plotted once the maximum absorbance peak was obtained and this was used to calculate the adsorbent's adsorption capacity. For the adsorption of dye on AgNPs, varying dye concentrations ranging from 5 mg/L to 2 mg/L were employed. Plotting concentration against incubation time allowed for the evaluation of the adsorbent's adsorption capacity. For ten hours at regular intervals, the absorbance of an adsorbent containing dye at various concentrations was measured. Figure 1 shows the schematic representation of the experiment (Figure 1).
Figure 1: Schematic representation of the experiment.
RESULTS AND DISCUSSION
AgNPs characterization those were stored
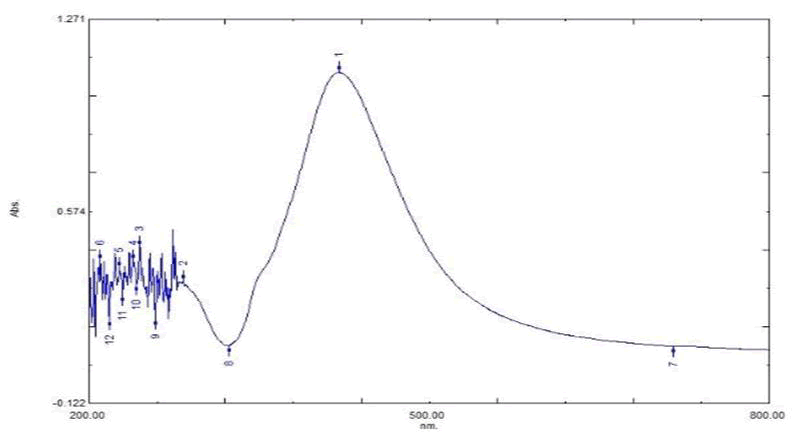
AgNPs were characterized using TEM, FTIR, PSA, zeta potential and UV-Vis spectroscopy. As indicated in Figure 3, the absorbance peak at 425 nm in the UV-Vis results validates the presence of silver in the sample. The sample was subsequently exposed to PSA, which confirms thatthe samples were in particle size range of 47 nm AgNPs, as indicated in Figure 4. Zeta potential results showed a negative value of -19.9 mV, indicating that the stored nanoparticles have a good colloidal nature, are highly dispersible and are stable over an extended period of time (Figure 5). The TEM results effectively verified that the particles, as depicted in Figure 2, are spherical in shape and in the nano range. Figure 6 signature AgNP peaks, which are located approximately at 671, 721, 1317, 1648, 1867 and 1919 cm-1, were revealed by FTIR analysis. The synthesized nanoparticles are active and show good characteristics of nanoparticles that can be successfully used to degrade the toxic dyes, as demonstrated by all these characterization techniques (Figures 2-6).
Figure 2: UV-Vis peak of AgNPs (a) and transmission electron microscopic image of the Ag NPs (b) during synthesis.
Figure 3: AgNPs- storage at 4ºC- UV-Vis absorbance peak.
Figure 4: PSA results of the AgNPs post storage.
Figure 5: Zeta potential of AgNPs after storage at 4ºC.
Figure 6: FTIR-stored sample of AgNPs.
Discoloration of methylene blue dye
Maximum absorbance in aqueous solution of methylene blue dye was found to be at 620 nm. A graph was plotted for varying concentrations of methylene blue dye as seen in Figure 7.
Different concentrations were prepared from common stock solution of the dye. Pre-synthesized AgNPs were utilized in an adsorption study with methylene blue at four different concentrations 5 mg/L, 4 mg/L, 3 mg/L and 2 mg/L. For the adsorption study, 5 mg of adsorbent was added to the various methylene blue concentrations previously mentioned. As illustrated in Figure 8, the highest amount of adsorption was noted at 3 mg/L concentration, at which point the dye completely lost its colour in 6 hours. The adsorbent needed longer incubation times at the other three concentrations to remove dye more effectively. This might be the result of dye saturation on the nanoparticle surface, which reduce the particles ability to adsorb dye (Figures 7 and 8).
Figure 7: Graph depicting different concentrations of methylene blue.
Figure 8: Stacked line graph illustrating adsorption of dye at varied concentrations of methylene blue.
CONCLUSION
The current technique effectively reported the use of silver nanoparticles for adsorption-based dye removal in an aqueous medium. FTIR, PSA, zeta potential, UV-Vis and other characterization methods all verified the activity of the pre-synthesised silver nanoparticles. Ag NPs provided a lot of surface area for the methylene blue dye to break down upon. Because of their extremely lowered zeta potential value, it was found that nanoparticles in the size range of about 46 nm easily dispersed in solutions rich in dye. This keeps the nanoparticles in the aqueous medium extremely stable. It was observed and recorded that the color of the methylene blue dye diminished in less than 6 hours after being exposed to a concentration of 3 mg/L of nanoparticles. Hence, this process adequately proves the efficacy of dye removal from the textile and other industrial effluents.
References
- Martins N, et al. Trends Food Sci Technol. 2016;52:1-5.
- Nautiyal P, et al. J Environ Manag. 2016;182:187-197.
[Crossref] [Google Scholar] [PubMed]
- Alves de Lima, et al. Mutat Res/Genet Toxicol Environ Mutagen. 2007;26(1-2):53-60.
[Crossref] [Google Scholar] [PubMed]
- Javaid R, et al. Int J Environ Res Public Health. 2019;16(11):2066.
[Crossref] [Google Scholar] [PubMed]
- Saleh SM. Spectrochim Acta A Mol Biomol Spectrosc. 2019;211:141-147.
[Crossref] [Google Scholar] [PubMed]
- Ruhl AS, et al. Chem Eng J. 2014;257:184-190.
- Gao X, et al. J Electroanal Chem. 2019;832:247-253.
- Punzi M, et al. J Hazard Mater. 2015;292:52-60.
[Crossref] [Google Scholar] [PubMed]
- GilPavas E, et al. J Environ Manag. 2017;191:189-197.
[Crossref] [Google Scholar] [PubMed]
- Wang T, et al. Chem Eng J. 2018;351:1013-1026.
- Korenak J, et al. J Clean Prod. 2019;210:1483-1495.
- Hameed BH, et al. Desalination. 2009;247(1-3):551-560.
- Dai L, et al. Bioresour Technol. 2018;267:510-516.
[Crossref] [Google Scholar] [PubMed]
- Maleki A, et al. Arab J Chem. 2020;13(1):3017-3025.
- Bulgariu L, et al. J Mol Liq. 2019;276:728-747.
- Tan CH, et al. S Afr J Chem Eng. 2018;26:11-24.
- Augustine R, et al. J Drug Deliv Sci Technol. 2020;56:101516.
- Abdel-Aziz R, et al. J Photochem Photobiol A Chem. 2020;389:112245.
- de Araujo Padilha CE, et al. Ind Crops Prod. 2020;146:112167.
- Reddy CV, et al. Chemosphere. 2020;239:124766.
[Crossref] [Google Scholar] [PubMed]