Original Articles: 2022 Vol: 14 Issue: 7
The Anti-diarrhea Activity of Ethanol and Aqueous Extract of Citrullus colocynths Pulp in Castor Oil and Magnesium Sulphate-Induced Diarrhea, Enter Pooling and Intestinal Transit Models
Asiat Na Allah, Mutiu A Alabi1*, Jamiu B Ahmad1, Fatai A Kareem2, Ayodeji O Obatoye3,Barakat Olorunshogo1, Rukayat Akinode1
1Department of Biochemistry, Kwara State University Malete, Kwara, Nigeria
2Department of Science Laboratory Technology, School of Science and Technology, Saapade, Ogun, Nigeria
3Department of Production, Federal Institute of Industrial Research, Oshodi, Lagos State, Nigeria
- Corresponding Author:
- Mutiu A Alabi
Department of Biochemistry,
Kwara State University Malete, Kwara, Nigeria
Received: 16-May-2022, Manuscript No. JOCPR-22-63906; Editor assigned: 18-May-2022, PreQC No. JOCPR-22-63906 (PQ); Reviewed: 01-Jun-2022, QC No. JOCPR-22-63906; Revised: 15-Jul-2022, Manuscript No.JOCPR-22-63906 (R); Published: 22-Jul-2022, DOI:10.37532/0975-7384.2022.14(7).031.
Citation: Farvardin M, Johari MK, Nami M, et al. Annular choroidal detachment one year after argus-II retinal prosthes is implantation.
Abstract
Diarrhea is a common health problem in developing nations, characterized by gut secretion and motility dysfunction. Nigerians tradopractitioners use a wide range of medicinal herbs to curb this menace, among is Citrullus colocynths. The pulp of the plant is used heavily in the treatment of diarrhea, hence the need to ascertain the efficacy of the plant as an anti-diarrhea agent. The anti-diarrhea activity of ethanol and aqueous extract of C. colocynths pulp (ECCP and ACCP respectively) was investigated using castor oil and magnesium sulphate (MgSO4) induced diarrhea, enter pooling and intestinal transit models. Control received distilled water (2 mL/kg, p.o.), diarrhea group received either castor oil or MgSO4 (2 mL/kg, p.o.), standard drug received loperamide (2mg/kg, p.o.), while treated groups received either ECCP or ACCP (50, 100 and 150 mg/kg, p.o. respectively). Incastor oil or MgSO4 diarrhea model, ECCP and ACCP significantly (p<0.05) prolonged the onset of diarrhea,decreased the total number and weight of diarrhea feaces corresponding to the increment seen in the % inhibitionof diarrhea feaces. Similarly, ECCP revealed a significant (p<0.05) reduction in gastrointestinal motility justifyingthe reduction seen in peristalsis index. The weight and volume of intestinal contents were observed to besignificantly (p<0.05) reduced in enter pooling and PGE-2 model of diarrhea. In conclusion, the results obtainedconfirmed the anti-diarrhea activities of ECCP and ACCP and therefore provide scientific basis for the use of thisplant traditionally by tradopractitioners.
Keywords
Diarrhea; Citrullus colocynths; Castor oil; Magnesium sulphate; Enter pooling
Introduction
Diarrhea is a disorder characterized by passage of semi-solid or watery fecal matter for three or more times in a day with increase in gastrointestinal motility and secretion coupled with a decrease in fluid and electrolyte absorption [1]. Diarrhea affects mainly children and infants and is one of the leading causes of preventable death in developing countries [2]. It generally requires no treatment but in severe cases, dehydration and electrolyte imbalance are the major risks, hence requiring both pharmacologic and non-pharmacologic treatments. The treatment of diarrhea is non-specific and is usually aimed at reducing the discomfort and inconvenience of frequent bowel movements [3]. Several African medicinal plants have been reported to be useful in the treatment, management and/or control of diarrhea [4-6].
Medicinal plants play a major role in the human health care, for this reason, it encouraged studies pertaining to tradomedicine in the treatment and prevention of diarrhea [2-7]. Available orthodox drugs are linked with contraindications and adverse effects [8], hence the need for herbal medicinal remedy in the management of diarrhea.
Citrullus colocynths plant belongs to the family Cucurbitaceae and is known as bitter apple, bitter cucumber, egusi [9]. It is a viny plant mainly found in Mediterranean Europe, Asia, Turkey, Nubia, Trieste, Egypt, Iran, Pakistan, Afghanistan, India and North Africa. Seeds contain a big range of fatty acids like stearic acid, myristic acid, palmitic acid, oleic acid, linoleic acid and linolenic acid [10,11]. The plant is rich in amino acids such as lysine, leucine and methionine. It also contains vitamins B1, B2 and niacin. Minerals in form of calcium, magnesium, potassium, iron and manganese are also present [12,13]. The aerial and fruit part contain flavonoid glycoside quercetin, flavone-3-glucoside viz iso-vitexin, iso-orentine and iso-orentine-3-methyl ether [14,15]. The fruit has been reported to be rich in cucurbitane type triterpine glycoside viz colocynthoside A and B. Cucurbitane type triterpene glycoside ciz cucurbitacin E 2-O-beta-D-glycoside, its aglycone cucurbitacin E and 2-O-beta-D-glycopyranosyl-cucrbitacin B and 2,25-di-O-beta-D-glycopyronasyl-cucurbitacin L [10,16]. Ethnobotanical study reports anti-inflammatory effect [17], anti-oxidative effect [18], anticonvulsant effect [19,20] anti-alopecia effect, anti-fungal effect and anti-diabetic effect. In our study, anti-diarrhea activities of ethanol and aqueous extracts of Citrullus colocynths pulp were studied.
Castor oil is a triglyceride characterized by a high content of hydroxylated unsaturated fatty acid ricinoleic acid ((9Z, 12R)-12-hydroxyoctadec-9-enoic acid). After oral ingestion of castor oil, ricinoleic acid is released which induces a strong laxative effect. Magnesium sulphate on the other hand, induces diarrhea by promoting cholecytokinin release from the duodenal mucosa preventing the reabsorption of sodium chloride and water from the lumen.
Materials and Methods
Experimental design
Chemicals: Loperamide, castor oil, activated charcoal, prostaglandin E2 (Sigma-Aldrich Chemical Pvt. Ltd., USA), distilled water, sodium chloride, magnesium sulphate and all other reagents used were of analytical grade.
Animals: Two hundred and ten (210) male Wistar rats (160-200 g) were obtained from the Animal House of Biochemistry Department of Kwara State University, Malete and were maintained on standard animal pellets and water ad libitum. They were acclimatized to laboratory conditions for three days before the commencement of administration. The approvals for animal studies were obtained from the University Animal Ethics Committee, Kwara State University and Malete.
Collection of plant material and identification: The whole fruit of Citrullus colocynths plant was purchased from local herb sellers at Idi-ape Market, Ilorin, Kwara State, Nigeria. The plant was identified and authenticated at the Department of Plant and Environmental Biology, University of Ilorin, Kwara State. A voucher number specimen (UILH/001/2020) was deposited at the Departmental Herbarium.
Preparation and extraction of the plant material: The whole fruit was peeled in other to remove the pulp which was then cut into pieces with the aid of a sterile knife. Thereafter, the fruit pulp was oven dried at a temperature of 45 to 50°C. After drying, the pulp of the fruit was pulverized into powder using a high speed electric blender. Equal amount of the powdered pulp was extracted into ethanol and distilled water with continuous agitation for 24 h. The resulting solution was sieved with a sterile muslin cloth and subsequently filtered using Whatman filter paper no 1. The Ethanol extract of C. colocynths Pulp (ECCP) filtrate was evaporated in a rotary evaporator while the Aqueous extract of C. colocynths Pulp (ACCP) filtrate was concentrated to dryness using water bath at 40°C. The extracts were refrigerated prior to use.
Anti-diarrhea Activity: The anti-diarrhea efficacy of extracts of C. colocynths pulp was assessed using either; castor oil or MgSO4 induced diarrhea, castor oil or MgSO4 induced enter pooling and charcoal meal and prostaglandin E2 intestinal transit models.
Castor oil-induced diarrhea model: Diarrhea was induced in rats according to the previously described method [28,29] with slight modifications. The rats were fasted for 18 h prior to the experiment but had access to clean water. Forty-five (45) rats were randomly divided into nine (9) groups of five animals each. Group 1 (control) received distilled water (2 mL/kg, p.o.), group 2 (castor oil) received distilled water (2 mL/kg, p.o.), group 3 (standard drug) received loperamide (2 mg/kg, p.o.), groups 4-6 received ECCP (50, 100 and 150 mg/kg, p.o. respectively), groups 7-9 received ACCP (50, 100 and 150 mg/kg, p.o. respectively). After 1 h, diarrhea was induced in group 2 and other treatment groups (groups 3-9) with the administration of castor oil (2 mL/kg, p.o.) and was separated in different cages lined with Whatman paper of uniform weights to collected feaces. The watery fecal material and frequency of defecation was noted for 4 h duration and total number of wet or unformed droppings was counted. Diarrhea was determined based on the presence of fluid in the fecal material, the mean stool number and diarrhea stool per group was determined and compared with the control groups. The results were expressed as percentage of inhibition of defecation and diarrhea respectively using the expression:
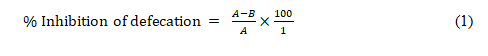
Where A=mean number of defecation caused by castor oil and B= mean number of defecation caused by drug or extract.
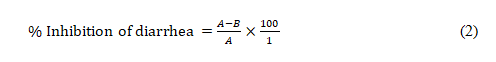
Where A= mean weight of fecal material by castor oil and B= mean weight of fecal material in drug/extract.
Castor oil-induced enter pooling activity: This method was used to determine the ability of the plant extract to prevent accumulation of fluid in castor oil induced enter pooling. Castor oil-induced enter pooling activity was determined as previously described [20] with slight modification. The rats were fasted for 18 h prior to the experiment but had access to clean water. Thirty (30) rats were randomly divided into six (6) groups of five animals each. Group 1 (control) received distilled water (2 mL/kg, p.o.), Group 2 (castor oil) received distilled water (2 mL/kg, p.o.), group 3 (standard drug) received loperamide (2 mg/kg, p.o.), groups 4-6 received ECCP (50, 100 and 150 mg/kg, p.o. respectively). After 1 h, diarrhea was induced in group 2 and other treatment groups (groups 3-6) with the administration of castor oil ((2 mL/kg, p.o.). The animals were euthanized 2 h later though cervical dislocation. The whole length of the intestine from the pylorus to the caecum was collected and the content of the small intestine was collected in the measuring cylinder. The volume and the weight of the intestinal content were measured and recorded (in cm3 and gram) respectively.


Castor oil-induced gastrointestinal motility (Charcoal Test): The animals were fasted for 18 h prior to the experiment but had access to clean water as previously described [18] with slightly modification. Thirty (30) rats were randomly divided into six (6) groups of five animals each. Group 1 (control) received distilled (2 mL/kg, p.o.), group 2 (castor oil) received distilled water (2 mL/kg, p.o.), group 3 (standard drug) received loperamide (2 mg/kg, p.o.), groups 4-6 received ECCP (50, 100 and 150 mg/kg, p.o. respectively). Thirty mins later, animals in groups 2-6 were administered activated charcoal (2 mL of 10% charcoal in 5% gum tragacanth, p.o.). The animals were then euthanized 30 mins later and the distance travelled by the charcoal meal from the pylorus to the caecum was measured and expressed as percentage of distance travelled using the expression:
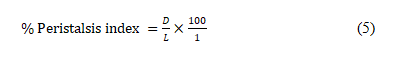
Where D is the distance travelled by the charcoal meal (m) and L is the intestinal length (m).
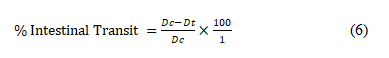
Where Dc is the mean distance travelled by diarrhea and Dt is the mean distance travelled by drug/extract group.
Prostaglandin E2 (PGE2) enter pooling Test: Prostaglandin E2 (PGE2)-enter pooling test was determined as previously described. Animals were grouped and dosed as described in charcoal test. An hour later, animals in groups 2-6 were administered prostaglandin E2 (1 mL of 100 μg/kg PGE2 in 5% gum tragacanth, p. o.). The animals were then euthanized 30 mins later and assessment of accumulation of intestinal secretions induced by (PGE2) from the pylorus to the caecum was measured and expressed as percentage of distance travelled.
Magnesium Sulphate (MgSO4)-induced diarrhea model: In this model, diarrhea was induced in rats according to the previously described method, with slight modifications. The rats were fasted for 18 h prior to the experiment but had access to clean water. Forty-five (45) rats were randomly divided into nine (9) groups of five animals each. All groups were dosed and treated as earlier outlined in castor-oil induced diarrhea model. The results were expressed as percentage of inhibition of defecation and diarrhea respectively.
Magnesium Sulphate-induced enter pooling activity: The ability of the plant extracts to prevent accumulation of fluid was also determined in this model as previously described. The rats were fasted for 18 h prior to the experiment but had access to clean water. Thirty (30) rats were randomly divided into six (6) groups of five animals each. All groups were dosed and treated as earlier outlined in castor-oil induced enter pooling model. The whole length of the intestine from the pylorus to the caecum was collected and the content of the small intestine was collected in the measuring cylinder. The volume and the weight of the intestinal content were measured and recorded (in cm3 and gram) respectively Table 1.
| S/N | Peak | Area | R.T | Height | Name of Compounds | MW | MF |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 0.13 | 5.311 | 0.3 | 1-Octene, 3-(methoxy methoxy) | 172 | C10H20O2 |
| 2 | 2 | 0.42 | 5.902 | 0.43 | (2,3-Dimethyloxiranyl)methanol | 102 | C5H10O2 |
| 3 | 3 | 0.45 | 6.087 | 0.81 | 2-Heptanol, acetate | 158 | C9H18O2 |
| 4 | 4 | 0.26 | 6.288 | 0.45 | 6(H),7(H)-Pyrazolo[4,5-E]1,2,4]triazin-5-one | 137 | C4H3N5O |
| 5 | 5 | 1 | 6.5 | 0.55 | 2-Deoxy-D-galactose | 164 | C6H12O5 |
| 6 | 6 | 0.75 | 6.811 | 0.69 | Isosorbide Dinitrate | 236 | C6H8N2O8 |
| 7 | 7 | 0.92 | 6.946 | 1.43 | 1,6-Octadien-3-ol, 3,7-dimethy | 154 | C10H18O |
| 8 | 8 | 6.19 | 7.781 | 1.19 | 1-Diisopropylsilyloxybutane | 188 | C10H24OS |
| 9 | 9 | 0.3 | 8.135 | 0.29 | Heptanoic acid, 6-hydroxy | 174 | C9H18O3 |
| 10 | 10 | 0.44 | 8.414 | 0.31 | 4H-Pyran-4-one, 3,5-dihydroxy-2-methyl | 142 | C6H6O4 |
| 11 | 11 | 0.69 | 8.716 | 0.34 | 4-Pyrimidinol, 5-methoxy- | 126 | C5H6N2O2 |
| 12 | 12 | 0.23 | 9.175 | 0.23 | Bicyclo[4.1.0]heptan-3-ol, 4,7,7-trimethyl-, [1R-(1.alpha.,3.alpha.,4.beta.,6.alpha.)] | 154 | C10H18O |
| 13 | 13 | 0.26 | 9.316 | 0.26 | Silane, trimethyl(4-methyl-3-penten-1-ynyl) | 152 | C10H18O |
| 14 | 14 | 0.28 | 9.568 | 0.29 | 3-cis-Methoxy-5-cis-methyl-1R-cyclohexano | 144 | C8H16O2 |
| 15 | 15 | 0.2 | 9.802 | 0.28 | 2-Methoxy-4-vinylphenol | 150 | C9H10O2 |
| 16 | 16 | 1.03 | 10.625 | 0.57 | 2(1H)-Naphthalenone, octahydro-4a,7,7-trimethyl-, cis | 194 | C13H22O |
| 17 | 17 | 4.36 | 11.446 | 7.7 | Caryophyllene | 204 | C15H24 |
| 18 | 18 | 5.68 | 11.808 | 9.04 | Humulene | 204 | C15H24 |
| 19 | 19 | 1.49 | 12.223 | 4.42 | Cyclohexane, 1-ethenyl-1-methyl-2, 4-bis (1-methylethenyl)-, [1S-(1.alpha. 2. beta., 4.beta.)] | 204 | C15H24 |
| 20 | 20 | 0.26 | 12.377 | 0.66 | Toluene-4-sulfonic acid, 2,7-dioxatricyclo[4.3.1.0(3,8)]dec-10-yl ester | 310 | C15H18O5S |
| 21 | 21 | 0.18 | 12.609 | 0.44 | Propylphosphonic acid, fluoroanhydride, decyleste | 266 | C13H28FO2P |
| 22 | 22 | 0.4 | 12.966 | 0.82 | cis-Z-.alpha.-Bisabolene epoxide | 220 | C15H24O |
| 23 | 23 | 0.55 | 13.186 | 1.46 | 12-Oxabicyclo[9.1.0]dodeca-3,7-diene, 1,5,5,8-tetramethyl-, [1R-(1R*,3E,7E,11R*)]- | 220 | C15H24O |
| 24 | 24 | 0.35 | 13.224 | 0.55 | 3,5-Octadiene, 2,2,4,5,7,7-hexamethyl- | 194 | C14H26 |
| 25 | 25 | 0.49 | 13.382 | 0.97 | cis-Z-.alpha.-Bisabolene epoxide | 220 | C15H24O |
| 26 | 26 | 0.94 | 14.323 | 3.93 | Octadecanoic acid | 284 | C18H36O2 |
| 27 | 27 | 0.67 | 14.453 | 4.54 | [1,1'-Bicyclopropyl]-2-octanoic acid, 2'-hexyl-, methyl ester | 332 | C21H38O2 |
| 28 | 28 | 0.31 | 15.058 | 2.47 | 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione | 212 | C11H16O4 |
| 29 | 29 | 6.57 | 15.882 | 6.34 | n-Hexadecanoic acid | 256 | C16H32O2 |
| 30 | 30 | 1.68 | 15.973 | 2.69 | Hexadecanoic acid, ethyl ester | 284 | C18H36O2 |
| 31 | 31 | 0.92 | 16.6 | 4.25 | 17-Octadecynoic acid | 280 | C18H36O2 |
| 32 | 32 | 9.32 | 17.03 | 7.89 | 9,12-Octadecadienoic acid (Z,Z) | 280 | C18H36O2 |
| 33 | 33 | 1.11 | 17.079 | 1.64 | Oleic Acid | 282 | C18H36O2 |
| 34 | 34 | 2.18 | 17.133 | 3.91 | 9,9-Dimethoxybicyclo[3.3.1]nona-2,4-dione | 212 | C11H16O4 |
| 35 | 35 | 1.45 | 17.24 | 3.6 | Ethyl Oleate | 310 | C20H38O2 |
| 36 | 36 | 1.83 | 17.325 | 4.77 | Naphthalene, decahydro-1-pentadecyl | 348 | C25H48 |
| 37 | 37 | 1.79 | 17.4 | 4.62 | Naphthalene, 2-decyldecahydro- | 278 | C20H38 |
| 38 | 38 | 1.37 | 17.737 | 3.39 | 2,5-Octadiene, 3,4,5,6-tetramethyl- | 166 | C12H22 |
| 39 | 39 | 1.23 | 17.781 | 2.36 | Hexadecanal, 2-methyl- | 254 | C17H34O |
| 40 | 40 | 0.71 | 17.852 | 2.8 | 17-Octadecynoic acid | 280 | C18H32O2 |
| 41 | 41 | 7.17 | 18.045 | 13.08 | 3-Hexen-1-ol, 6-(2,6,6-trimethyl-1-cyclohexenyl)-4-methyl-, (E)- | 236 | C16H28O |
| 42 | 42 | 2.61 | 18.733 | 3.97 | Hexadecanal, 2-methyl- | 254 | C17H34O |
| 43 | 43 | 2.86 | 18.94 | 3.15 | Hexadecanoic acid, 2-hydroxy-1-(hydroxymethyl)ethyl ester | 330 | C19H38O4 |
| 44 | 44 | 1 | 19.12 | 4.42 | Cyclopropanenonanoic acid | 322 | C21H38O2 |
| 45 | 45 | 1.26 | 19.349 | 5.4 | 1-Heptadec-1-ynyl-cyclohexanol | 334 | C23H42O |
| 46 | 46 | 1.78 | 19.454 | 4.06 | Hexadecanoic acid,1-(hydroxymethyl)-1,2-ethanediyl este | 568 | C35H68O5 |
| 50 | 50 | 1.18 | 20.464 | 3.15 | Trilinolein | 878 | C57H98O6 |
| 51 | 51 | 1.41 | 20.496 | 2.35 | Oleoyl chloride | 300 | C18H33ClO |
| 52 | 52 | 4.12 | 20.663 | 6.7 | Beta.-Sitosterol | 414 | C29H50O |
| 53 | 53 | 1.06 | 20.8 | 4.92 | 2H-3,9 α -Methano-1-benzoxepin, octahydro-2,2,5 α,9-tetramethyl-, [3R-(3.alpha.,5a.alpha.,9.alpha.,9a.alpha.)] | 222 | C15H26O |
| 54 | 54 | 0.54 | 20.866 | 2.47 | 2,2,4-Trimethyl-3-(3,8,12,16-tetramethyl-heptadeca-3,7,11,15-tetraenyl)-cyclohexanol | 428 | C30H52O |
| 55 | 55 | 0.8 | 21.005 | 6.49 | 1-Heptadec-1-ynyl-cyclopentanol | 320 | C22H40O |
| 56 | 56 | 0.58 | 21.154 | 5.5 | 1-Heptadec-1-ynyl-cyclopentanol | 320 | C22H40O |
| 57 | 57 | 0.13 | 21.232 | 2.28 | 5-(1-Bromo-1-methyl-ethyl)-2-methyl-cyclohexanol | 234 | C10H19BrO |
| 58 | 58 | 0.82 | 21.364 | 6.16 | Menthol, 1'-(butyn-3-one-1-yl)-, (1R,2S,5R)- | 222 | C14H22O2 |
| 59 | 59 | 1.12 | 21.531 | 4.66 | 4-α-H-cycloprop[e]azulen-4 α -ol, decahydro-1,1,4,7-tetramethyl- | 222 | C15H26O |
RT=Retention Time; MW=Molecular Weight; MF=Molecular Formular.
Table 1: Bioactive compounds found in ethanol pulp extract of C. colocynths.
Statistical analysis: Data were analyzed using SPSS version 20 and the experimental results were expressed as mean ± Standard Error of the Mean (SEM). The statistical significance was carried out by employing one-way Analysis of Variance (ANOVA) followed by a Tukey post hoc test for multiple comparisons. The results were considered statistically significant when the p value is less than 0.05.
Results
Percentage yield of the ethanol and aqueous pulp extract: Approximately 14.07 and 65.55 g corresponding to 2.18 and 13.37% of ECCP and ACCP respectively were obtained.
Anti-diarrhea effect of ECCP and ACCP on castor oil-induced diarrhea: One h after administration of castor oil, animal’s revealed induction of diarrhea with frequent bowel discharge. Pre-treatment with the extracts (ECCP or ACCP) showed a significant reduction in number of defecation compared to the castor oil group. The mean number of diarrhea feaces in ECCP and ACCP were 22.98 ± 0.15, 25.14 ± 0.20, 15.80 ± 0.03 and 19.80 ± 0.12, 15.37 ± 0.90, 12.00 ± 0.01 at 50, 100 and 150 mg/kg doses respectively while in loperamide-treated group, the value is 11.20 ± 0.02. The % inhibition of defecation relative to the castor oil group was 47.53, 42.60, 63.93 % in ECCP treated group and 54.79, 65.75, 72.60 % in ACCP treated group respectively. Loperamide showed an inhibition of defecation (74.43 %) compared to the castor oil group (00.00 %). The mean weight of diarrhea stools in ECCP and ACCP were 3.00 ± 0.16, 3.90 ± 0.04, 2.40 ± 0.23 and 1.80 ± 0.31, 1.72 ± 0.02, 1.25 ± 0.18 at 50, 100 and 150 mg/kg doses respectively while in loperamide-treated group, the value is 1.00 ± 0.12. Percentage inhibition of wet feaces (diarrhea) was 58.45, 45.98 and 66.76 % and 75.07, 76.18, 82.69 % for ECCP and ACCP at 50, 100 and 150 mg/kg doses respectively. The inhibition recorded for loperamide treated group was 86.15 % (Table 2).
| Groups | Dose | Mean No. of Diarrhea Faeces | % Inhibition of Defecation | Weight of Diarrhea Faeces (mg) | % Inhibition of Diarrhea | Time Onset of Diarrhea (sec) |
|---|---|---|---|---|---|---|
| Control | 2.0 (ml/kg) | 5.00 ± 0.01a | 88.58 ± 0.00i# | 0.25 ± 0.00a | 96.54 ± 0.20i# | 2.05 ± 0.02b |
| Castor oil | 2.0 (ml/kg) | 43.80 ± 0.05h | 00.00 ± 0.00a | 7.22 ± 0.11i | 00.00 ± 0.00a | 0.06 ± 0.01a |
| Loperamide | 2 (mg/kg) | 11.20 ± 0.02b | 74.43 ± 0.02h# | 1.00 ± 0.12b | 86.15 ± 0.83h# | 2.21 ± 0.02d |
| Ethanol Extract Aqueous Extract |
50 (mg/kg) | 22.98 ± 0.15f 19.80 ± 0.12e |
47.53 ± 0.01c# 54.79 ± 0.01d# |
3.00 ± 0.16f 1.80 ± 0.31d |
58.45 ± 0.58c# 75.07 ± 0.45e# |
2.28 ± 0.01d 2.02 ± 0.01b |
| Ethanol Extract Aqueous Extract |
100 (mg/kg) | 25.14 ± 0.20g# 15.37 ± 0.90d# | 42.60 ± 0.02b# 65.75 ± 0.02f# |
3.90 ± 0.04h 1.72 ± 0.02h |
45.98 ± 0.07b# 76.18 ± 0.24f# |
2.43 ± 0.01e 2.12 ± 0.01c |
| Ethanol Extract Aqueous Extract |
150 (mg/kg) | 15.80 ± 0.03d 12.00 ± 0.01c |
63.93 ± 0.01e# 72.60 ± 0.03g# |
2.40 ± 0.23e 1.25 ± 0.18c |
66.76 ± 0.17d# 82.69 ± 0.76g# |
2.42 ± 0.01e 2.01 ± 0.04b |
Values are expressed as mean ± SEM (n=6); b-ip<0.05 compared with control; #% inhibition is relative to castor-oil group.
Table 2: Effect of ethanol and aqueous extract of C. colocynths pulp in castor oil-induced diarrhea rat
Anti-diarrhea effect of ECCP on castor oil-induced enter pooling in rat: Administration of castor oil produced a significant (p<0.05) increase in intestinal fluid accumulation in castor oil group compared with control group. The mean weight of intestinal content of ECCP was 0.73 ± 0.04, 0.67 ± 0.03 and 0.66 ± 0.07 g while the volume of intestinal content was 0.80 ± 0.18, 0.70 ± 0.14 and 0.50 ± 0.14 cm3 respectively. The highest dose (150 mg/kg) revealed a significant reduction in both the weight and volume of the intestinal content. Compared with the castor oil group, the three doses of ECCP significantly (p<0.05) inhibited castor oil induced enter pooling in rats as revealed by higher percentage inhibition of intestinal weight content (81.98, 83.46 and 83.70 %) and intestinal volume content (73.33, 76.67 and 83.33 %) respectively (Table 3).
| Groups | Dose | Weight of Intestinal Content (g) | % Inhibition of Molecular Weight of Small Intestinal Content |
Volume of Intestinal Content (cm3) | % Inhibition of Molecular Volume of Small Intestinal Content |
|---|---|---|---|---|---|
| Control | 2 (ml/kg) | 0.05 ± 0.01a | 98.77 ± 0.20f# | 0.10 ± 0.11d | 96.67 ± 0.10g# |
| Castor oil | 2 (ml/kg) | 4.05 ± 0.05d | 00.00 ± 0.00a | 3.00 ± 0.21f | 00.00 ± 0.00a |
| Loperamide | 2 (mg/kg) | 0.97 ± 0.01c | 76.05 ± 0.16b# | 1.56 ± 0.21e | 48.00 ± 0.11b# |
| Ethanol Extract | 50 (mg/kg) | 0.73 ± 0.04b | 81.98 ± 0.25c# | 0.80 ± 0.18c | 73.33 ± 0.11d# |
| Ethanol Extract | 100 (mg/kg) | 0.67 ± 0.03b | 83.46 ± 0.27d# | 0.70 ± 0.14b | 76.67 ± 0.21e# |
| Ethanol Extract | 150 (mg/kg) | 0.66 ± 0.07b | 83.70 ± 0.25e# | 0.50 ± 0.14a | 83.33 ± 0.11f# |
Values are expressed as mean ± SEM (n=6); b-dp<0.05 compared with control; #% inhibition is relative to castor-oil. group.
Table 3: Effect of ethanol extract of C. colocynths pulp on castor oil-induced enter pooling in rat
Anti-diarrhea effect of ECCP on the intestinal transit of charcoal meal in rat: ECCP significantly (p<0.05) reduced castor oil induced gastrointestinal movement of charcoal meal compared with the castor oil group. Significant elevation (p<0.05) in % intestinal transit of charcoal meal was seen in 50 mg/kg (28.00 %) resulting in a better effect than loperamide (23.50%) relative to the castor oil (0.00 %) group. Compared to the control group, the percentage inhibition of intestinal transit was 28.77, 23.00 and 27.61 at 50, 100 and 150 mg/kg doses of the extract, respectively (Table 4).
| Groups | Dose | Mean length of small intestine (cm) | Mean distance travelled by charcoal meal (cm) |
Peristalsis index (%) | % Transit inhibition |
|---|---|---|---|---|---|
| Control | 2 (ml/kg) | 47.40 ± 5.96a | 34.11 ± 0.00a | 71.96 ± 0.09b | 52.91 ± 0.19e# |
| Castor oil | 2 (ml/kg) | 85.78 ± 0.02f | 72.44 ± 0.14e | 84.45 ± 0.03f | 00.00 ± 0.00a |
| Loperamide | 2 (mg/kg) | 69.60 ± 5.39d | 55.42 ± 0.03d | 79.63 ± 0.04d | 23.50 ± 0.55b# |
| Ethanol Extract | 50 (mg/kg) | 64.00 ± 5.70c | 51.60 ± 0.18b | 80.63 ± 0.08e | 28.77 ± 0.10d# |
| Ethanol Extract | 100 (mg/kg) | 72.80 ± 7.00e | 55.78 ± 0.07d | 76.62 ± 0.04c | 23.00 ± 0.08b# |
| Ethanol Extract | 150 (mg/kg) | 61.40 ± 4.30b | 52.44 ± 0.14c | 64.42 ± 0.03a | 27.61 ± 0.18c# |
Values are expressed as mean ± SEM (n=6); b-fp< 0.05 compared with control; #% inhibition is relative to castor-oil group.
Table 4: Effect of ECCP on intestinal transit of charcoal meal in rat
Anti-diarrhea effect of ECCP on PGE2-induced enters pooling in rat: Administration of PGE2 produced a significant (p<0.05) increase in intestinal fluid accumulation in PGE2 group compared with control group. The mean weight of intestinal content of ECCP were 1.92 ± 0.04, 0.99 ± 0.01 and 0.93 ± 0.01 g while the volume of intestinal content was 1.90 ± 0.12, 1.90 ± 0.12 and 1.00 ± 0.09 cm3 respectively. The highest dose (150 mg/kg) revealed a significant reduction in both the weight and volume of the intestinal content. Compared with the PGE2-induced group, the three doses of ECCP significantly (p<0.05) inhibited PGE2 induced enter pooling in rats as revealed by higher percentage inhibition of intestinal weight content (34.25, 66.10 and 68.15 %) and intestinal volume content (65.45, 65.45 and 81.81 %) respectively (Table 5).
| Groups | Dose | Weight of Intestinal Content (g) | % Inhibition of Molecular Weight of Small Intestinal Content |
Volume of Intestinal Content (cm3) | % Inhibition of Molecular Volume of Small Intestinal Content |
|---|---|---|---|---|---|
| Control | 2 (ml/kg) | 0.56 ± 0.01a | 80.82 ± 0.24f | 0.20 ± 0.05a | 96.36 ± 0.37e |
| PGE2 | 2 (ml/kg) | 2.92 ± 0.02e | 00.00 ± 0.00a | 5.50 ± 0.14e | 00.00 ± 0.00a |
| Loperamide | 2 (ml/kg) | 1.60 ± 0.02c | 58.90 ± 0.11c | 1.60 ± 0.11c | 70.91 ± 0.14c |
| ethanol extract | 50 (mg/kg) | 1.92 ± 0.04d | 34.25 ± 0.11b | 1.90 ± 0.12d | 65.45 ± 0.05b |
| ethanol extract | 100 (mg/kg) | 0.99 ± 0.01b | 66.10 ± 0.11d | 1.90 ± 0.12d | 65.45 ± 0.05b |
| ethanol extract | 150 (mg/kg) | 0.93 ± 0.01b | 68.15 ± 0.09e | 1.00 ± 0.09b | 81.81 ± 0.11d |
Values are expressed as mean ± SEM (n=6); b-ep<0.05 compared with control; #% inhibition is relative to PGE2 group.
Table 5: Effect of ECCP on intestinal transit of PGE2 in rat
Anti-diarrhea effect of ECCP and ACCP on magnesium sulphate-induced diarrhea: Animals revealed induction of diarrhea with frequent bowel discharge 1 h post MgSO4 administration. Pre-treatment with either ECCP or ACCP revealed a significant reduction in number of defecation compared to the MgSO4 induced group. The mean number of feaces in ECCP and ACCP were 19.80 ± 0.11, 18.75 ± 0.08, 15.80 ± 0.08 and 17.00 ± 0.08, 15.00 ± 0.06, 12.00 ± 0.13 at 50, 100 and 150 mg/kg doses respectively while in loperamide-treated group, the value is 10.40 ± 0.03. The % inhibition of defecation relative to the MgSO4-induced group was 68.67, 43.52, 52.41 % in ECCP treated group and 48.80, 54.82, 63.86% in ACCP treated group respectively. Loperamide showed an inhibition of defecation (63.21%) compared to the MgSO4 induced group (00.00%). The mean weight of diarrhea stools in ECCP and ACCP were 2.80 ± 0.18, 1.70 ± 1.37, 2.40 ± 0.23 and 1.70 ± 0.07, 1.67 ± 0.24, 1.67 ± 0.32 at 50, 100 and 150 mg/kg doses respectively while in loperamide-treated group, the value is 1.10 ± 0.02. Percentage inhibition of wet feaces (diarrhea) was 57.70, 74.32, 63.75 % and 74.32, 74.77, 74.77% for ECCP and ACCP at 50, 100 and 150 mg/kg doses respectively. The inhibition recorded for loperamide-treated group was 83.38 % (Table 6).
| Groups | Dose | Mean No. of Diarrhea Faeces | % Inhibition of Defecation | Weight of Diarrhea Faeces |
% Inhibition of Diarrhea |
|---|---|---|---|---|---|
| Control | 2 (ml/kg) | 7.00 ± 0.04a | 78.92 ± 0.00h# | 1.00 ± 0.01a | 84.89 ± 0.19f# |
| MgSO4 | 2 (ml/kg) | 33.20 ± 0.17h | 00.00 ± 0.00a | 6.62 ± 0.13f | 00.00 ± 0.00a |
| Loperamide | 2 (mg/kg) | 10.40 ± 0.03b | 63.21± 0.24f# | 1.10 ± 0.02b | 83.38 ± 0.22e |
| Ethanol extract Aqueous extract |
50 (mg/kg) | 19.80 ± 0.11g 17.00 ± 0.08e |
68.67 ± 0.13g# 48.80 ± 0.10c# |
2.80 ± 0.18e 1.70 ± 0.07c |
57.70 ± 0.61b 74.32 ± 0.35d |
| Ethanol extract Aqueous extract |
100 (mg/kg) | 18.75 ± 0.08f 15.00 ± 0.06d |
43.52 ± 0.09b# 54.82 ± 0.06e# |
1.70 ± 1.37c 1.67 ± 0.24c |
74.32 ± 0.12d 74.77 ± 0.55d |
| Ethanol extract Aqueous extract |
150 (mg/kg) | 15.80 ± 0.08d 12.00 ± 0.13c |
52.41 ± 0.07d# 63.86 ± 0.05f# |
2.40 ± 0.23d 1.67 ± 0.32c |
63.75 ± 0.09c 74.77 ± 0.26d |
Table 6: Effect of ethanol and aqueous extract of C colocynths pulp on MgSO4-induced diarrhea in rat
Anti-diarrhea effect of ECCP on magnesium sulphate-induced enters pooling: Magnesium sulphate administration produced a significant (p<0.05) increase in the accumulation of intestinal fluid in MgSO4 group compared with control group. The mean weight of intestinal content of ECCP was 0.78 ± 0.02, 0.70 ± 0.01 and 0.66 ± 0.03 g while the volume of intestinal content was 1.49 ± 0.11, 0.90 ± 0.04 and 0.46 ± 0.03 cm3 respectively. The highest dose (150 mg/kg) revealed a significant reduction in both the weight and volume of the intestinal content. Compared with the MgSO4 group, the three doses of ECCP significantly (p<0.05) inhibited MgSO4-induced enter pooling in rats as revealed by higher percentage inhibition of intestinal weight content (71.92, 74.82 and 76.25 %) and intestinal volume content (66.44, 79.73 and 89.64 %) respectively (Table 7).
| Groups | Dose | Weight of intestinal content (g) | % Inhibition of molecular weight of small intestinal content | Volume of intestinal content (cm3) | % Inhibition of molecular volume of small intestinal content |
|---|---|---|---|---|---|
| Control | 2 (ml/kg) | 1.07 ± 0.01d | 61.51 ± 0.50b# | 1.60 ± 0.09e | 62.50 ± 0.25b# |
| MgSO4 | 2 (ml/kg) | 2.78 ± 0.02e | 00.00 ± 0.00a | 4.44 ± 0.03f | 00.00 ± 0.00a |
| Loperamide | 2 (mg/kg) | 1.00 ± 0.06c | 64.03 ± 0.43c# | 0.80 ± 0.08b | 81.98 ± 0.55e# |
| Ethanol Extract | 50 (mg/kg) | 0.78 ± 0.02b | 71.92 ± 0.18d# | 1.49 ± 0.11d | 66.44 ± 0.40c# |
| Ethanol Extract | 100 (mg/kg) | 0.70 ± 0.01b | 74.82 ± 0.37e# | 0.90 ± 0.04c | 79.73 ± 0.48d# |
Values are expressed as mean ± SEM (n = 6); b-fp<0.05 compared with control; #% inhibition is relative to MgSO4 group.
Table 7: Effect of ethanol extract of C colocynths pulp on MgSO4 induced enter pooling in rat
Discussion
The study used different diarrhea models to evaluate the anti-diarrhea activity of ethanol and aqueous extract of C. colocynths. Diarrhea was induced by liberation of ricinoleic acid though the action of lipases in the small intestine by binding to EP-3 prostanoid receptors on smooth muscles facilitating the accumulation of fluid in the intestine thereby inhibiting absorption and improving fluid and electrolyte secretion [36-38] in castor oil model while magnesium sulfate model promotes the release of Cholecystokinin (CCK) from the duodenal mucosa leading to water and sodium chloride reabsorption in the lumen [17].
A total number of 51 compounds were identified from GC-MS analysis of ethanol extract of C. colocynths, majority of which are fatty acids (Table 1). Among the identified compounds are caryophyllene, humulene, toluene-4-sulfonic acid, 2,7-dioxatricyclo[4.3.1.0(3,8)]dec-10-yl ester, β-Sitosterol and 2-Methoxy-4-vinylphenol which are presumed to be responsible for eliciting therapeutic activity.
In the castor oil induced diarrhoea model, both ECCP and ACCP significantly displayed anti-diarrhea activity on all parameters measured: onset of diarrhoea, the number and weight of fecal material. This result is in concordance with the report on the evaluation of the anti-diarrheal activity of the aqueous stem extract of Lantana camara Linn in mice and comparative anti-diarrheal and anti-ulcer effect of the aqueous and ethanolic stem bark extracts of Tinospora cordifolia in rats [17]. Studies have reported the anti-inflammatory and analgesic activities of medicinal plants due to inhibition of prostaglandin biosynthesis as non-steroidal anti-inflammatory drugs does, thus, the anti-diarrhea action exerted by both ECCP and ACCP may also be attributed to the inhibition of prostaglandin [20]. This attribute is validated by the fact that castor oil, magnesium sulphate and prostaglandin E2 induced diarrhea are related to stimulation of prostaglandin synthesis.
Similarly, anti-diarrhea agents work by reducing propulsive movement of GI smooth muscle, hence inhibiting diarrhea. Mechanism for evaluating anti-diarrhea activity of the extract was also achieved via enter pooling and motility test. In the enter pooling model, ECCP significantly reduced fluid accumulation when compared with castor oil/MgSO4/PGE2 group. This effect was similar to what was observed in loperamide treated group, a widely known anti-diarrhea drug. Ricinoleic acid, an active metabolite of castor oil induces irritation and inflammation of the intestinal mucosa leading to release of prostaglandins thus stimulating its secretion thereby enhancing sodium chloride and water re-absorption. The inhibitory effect shown by ECCP may be due to inhibition of gastrointestinal hypersecretion via suppressing the inducer-stimulated prostaglandin biosynthesis and enter pooling thereby preventing water and electrolytes re-absorption. This result is comparable with an analogous study previously conducted [19].
The charcoal motility test was done to ascertain the gastrointestinal motility content because decrease in gastrointestinal content is one of the mechanisms by which anti-diarrhea agent acts. In the intestinal transit charcoal test, ECCP inhibited/delayed intestinal motility as observed by the decrease in distance travelled by the charcoal marker in the intestine. This finding implies an anti-motility action as a mechanism of action of ECCP and suggest that ECCP decreases hyper-motility, favoring increase in transit time though intestinal muscle spasm suppression, thus extending the time for absorptive processes [20].
Conclusion
In summary, our study demonstrates that ECCP and ACCP contain pharmacologically active substances that possess significant anti-diarrhea activity in different models. These attributes therefore support the traditional use of this plant in folk medicine and can be claimed as a potential therapeutic option for effective management of diarrhea.
References
- Ezeja IM, Ezeigbo II, Madubuike KG, et al. Asian Pac J Trop Med. 2012;5(2):147-150.
- Mekonnen B, Asrie AB, Wubneh ZB, et al. Evid based Complement Altern Med. 2018;3037120.
- LL Brunton. The Pharmacological Basis of Therapeutics. 11th edition, McGaw-Hill, New York, 2008; 623–652.
- Suleiman MM, Dzenda T, Sani CA, et al. J Ethnopharmacol. 2008;116:125–130.
- Adeyemi OO and Akindele AJ. J Ethnopharmacol. 2008; 116:407–412.
- Mbagwu HOC, Adeyemi OO. J Ethnopharmacol. 2008; 116:16–20.
- Snyder JD and Merson MH. Bull World Health Organ. 1982;60(4):605–613.
- Bonner L. Pharmacy Today. 2019; 25(12), 23.
- Meybodi MSK. Asian J Res Reports Endocrinol. 2020;3(1): 25-33.
- Davidovich‐Rikanati R, Shalev L, Baranes N, et al. Yeast, 2015;32:103-114.
- Pravin B, Tushar D, Vijay P, et al. Int J Res Pharm Chem. 2013;3:46-53.
- Gurudeeban S, Satyavani K, Ramanathan T, et al. Asian J Plant Sci. 2010;9:394.
- Hussain AI, Rathore HA, Sattar MZ, et al. J Ethnopharmacol. 2014;155:54-66.
- Dastmalchi K, Dorman HD, Koşar M, et al. LWT-Food Sci Technol. 2007;40:239-248.
- Sharififar F, Moshafi MH, Mansouri SH, et al. Food Control. 2007;18:800-805.
- Song F, Dai B, Zhang HY, et al. J Asian Nat Prod Res. 2015;17:813-818.
- Onyeji CO, Igbinoba SI, Olayiwola G, et al. Drug Metab Lett. 2017;11:74-85.
- Bernard SA and Olayinka OA. J Med Plant Res. 2010;4:2821-2826.
- Kaushik U, Aeri V, Mir SR, et al. Pharmacogn Rev. 2015;9:12.
- Mehzadi S, Shojaii A, Pur SA, et al. J Evid Based Complement Alternat Med. 2016;21:31-35.
