Original Articles: 2022 Vol: 14 Issue: 7
Synthesis of ZnO/CuO Nanocomposite by Coprecipitation method for Photocatalytic Degradation of Methyl Orange
Laila M. Al-Harbi*, Thuraya O. Basudan
Department of Chemistry, King Abdul-Aziz University, Jeddah, Saudi Arabia
- Corresponding Author:
- Laila M. Al-Harbi
Department of Chemistry,
King Abdul-Aziz University,
Jeddah,
Saudi Arabia
Received: 22-Jun-2022, Manuscript No. JOCPR-22-70824; Editor assigned: 24-Jun-2022, PreQC No.JOCPR-22-70824 (PQ); Reviewed: 08-Jul-2022, QC No. JOCPR-22-70824; Revised: 15-Jul-2022, Manuscript No.JOCPR-22-70824(R); Published: 22-Jul-2022, DOI:10.37532/0975-7384.2022.14(7).038
Abstract
Fabrication of CuO, ZnO, and ZnO/CuO binary metal oxide nanocomposites with different molar ratio of Zn/Cu (3:1), (1:1), and (1:3) were carried out via a coprecipitation method. The improvement characteristics of ZnO/CuO nanocomposites and structure phases were characterized by X-Ray Diffraction (XRD). Crystallite size was obtained by the Scherrer equation. The morphology was determined by Scanning Electron Microscopy (SEM) images. Elemental composition was determined by energy dispersive X-ray EDX. Measurements and evaluation of the photocatalytic efficacy of the ZnO/CuO nanocomposites were deducted and observed by photodegradation of Methyl Orange dye (MO) under UV lamp illumination, monitored by UV-Vis spectrophotometer. The optimum concentration of ZnO/CuO (3:1 molar ratio) exhibited the highest degradation percentage (100%) under the UV lamp, which is demonstrated superior efficiency compared with the other various ratios and pure ZnO and CuO CuO, (1:1) ZnO/CuO, (1:3) ZnO/CuO and (3:1) ZnO/CuO for the degradation of methyl orange dye.
Keywords
ZnO/CuO nanocomposite; Binary metal oxides; Coprecipitation; Methyl orange; Photocatalytic degradation
Introduction
Organic dyes are widely used in industrial applications due to their ease and effectiveness, but in contrast, their polluted residues are frequently found in industrial waste water resulting from the production of factories and discharge of their effluent wastes into natural water, which is not desirable because they induce indirect hazards through the formation of harmful intermediates (which are more toxic than the original compounds) and release of their highly toxic, polluting by-products into the natural waters. Within the dyeing process, around 12% of these dyes deports waste, and about 20 % of this wastage steps inside the environment. Non-degradable organic molecules existence poses difficulty removal of pollutants [1]. Researchers have devoted their efforts to finding solutions to recycle, utilize and purify industrial wastewater by converting dyes products into harmless materials. For this purpose, dye degradation is an effective solution. The degradation process involves converting large hazardous molecules of dye into easily degradable, harmless small molecules such as water, carbon dioxide, and minerals as side products. Photocatalysis is a suitable method for degradation, transform dyes products into safe byproducts. Compared with other methods, photocatalysis is a highly efficient, and economically feasible process [2]. Many metal-oxide semiconductors have been broadly considered in the photocatalytic degradation field because of the captivating attributes: Photosensitivity, economical, unpolluted nature, and environmentally safe. Therefore, they are employed in the photocatalytic process to improve its performance. One of these metal oxides, ZnO nanoparticles. Due to its fascinating characteristics, ZnO has been widely utilized in some dye degradation due to its stability, high photosensitivity, and wide bandgap. But the only limitation of zinc oxide is its highly effective only under UV irradiation and absorbs an insignificant portion of the visible spectrum [3,4]. Researchers worked to develop the performance and efficiency of ZnO in several ways. Doping process is one of the manners which enhance the photocatalytic activity including modification and combination of photocatalyst with a different metal oxide. Modification of ZnO (n-type semiconductor, band gap 3.37 eV) with incorporate another metal oxide such as CuO (p-type semiconductor, band gap 1.27 eV) can be useful as it augments the surface area, reduces the bandgap (due to p-n heterojunction), and performs extending ZnO absorption range to visible spectrum region which enhance photocatalytic activity. Many methods were used to prepare ZnO/CuO composite one of them is coprecipitation [5]. In coprecipitation method different molar ratio of zinc and copper precursors were used at specific temperature. Gajendiran and Rajendran used 2.16 g of zinc acetate dihydrate with 2.21 g of copper acetate dihydrate for 120⁰C for 15 hr the final product has spherical shape [6]. Benxialix prepared ZnO-CuO composite by using 2:1 molar ratio of ZnCl2 and CuSO4 at 80⁰C for 24 hours [7] while Susmita et al. mixed ZnSO4 and CuCl2 at the same experimental conditions. The ZnO/CuO composites obtained [8] have nanoflower structure. Mekdes synthesized ZnO/CuO composites by mixing 0.272 g of zinc nitrate hexahydrate with 0.2497 g of copper nitrate pentahydrate for 80⁰C for 15 hr [9]. The ZnO/CuO composites synthesis by mixing zinc chloride and copper oxide in the presence of hexadecyltrimethylammonium bromide (CTAB) and sodium hydroxide as surfactants [10].
This work aimed to prepare different molar ratio of ZnO/CuO nanocomposites by simple coprecipitation method using low-cost material metal nitrate as precursors to evaluate their photocatalytic activity. The obtained products were characterized by X-Ray Diffraction (XRD) Energy Dispersive X-ray spectroscopy (EDX) and Scanning Electron Microscopy (SEM) images. Photocatalytic activity was measured by photocatalytic degradation of Methyl Orange dye (MO) under UV lamp illumination, monitored by UV-Vis spectrophotometer. The described method for the preparation of ZnO/CuO nanocomposites is simple, cheap, and highly effective for dye removal form wastewater.
Materials and Methods
Copper nitrate, Cu(NO3)2, 3H2O, (E.Merk), zinc nitrate, Zn (NO3)2, 6H2O, (Alpha Chemika), oxalic acid, (COOH)2, (Qualikems Fine Chemicals), absolute ethanol, C2H5OH, 99.8%, (Sigma-Aldrich), acetone, CH3COCH3, 99% (BDH), hydrochloric acid, HCl, (BDH), sodium hydroxide, NaOH, (Sigma-Aldrich). Usage of all materials was without purification. The obtained nanoparticles were characterized by X-Ray Diffraction (XRD) Energy Dispersive X-ray spectroscopy (EDX) Scanning Electron Microscopy (SEM). All these characterizations done in center of nanotechnology king Abdulaziz University.
Preparation of Photocatalysts
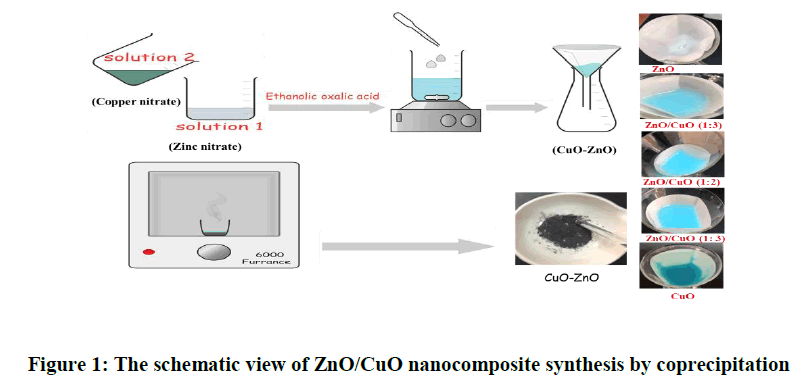
Various molar ratios of ZnO/CuO nanocomposite with different molar ratio of Zn/Cu (3:1), (1:1), and (1:3) respectively were prepared by mixing 20 ml of ethanolic (2.33,1.48,0.74 g) Zn (NO3)2.6H2O (Solution 1) with (0.6, 1.1,1,81 g) copper nitrate (Solution 2) drop wise with constant stirring. After mixing (1 M) ethanolic oxalic acid solution was added drop wisely with constant stirring. The precipitate was filtered, washed with ethanol, then acetone, and dried overnight in the air at room temperature. Precursor was calcined at 600℃ in a muffle furnace for 3 hours (Figure 1). Pure ZnO and CuO were prepared by the same procedure.
Photocatalytic Activity and Degradation Procedures
The photocatalytic degradation of MO was investigated in the presence of ZnO, CuO and various molar ratios of binary ZnO/CuO. 100 mg of each catalyst was added to 100 ml Methyl Orange (MO) aqueous solutions (1 ppm). After 1 hour in the dark, 5 ml was withdrawn, and then exposed the solutions to the UV lamp irradiation for 180 min. At a given time interval, 5 ml of the mixture was taken, centrifuged to separate the catalyst from the solution. The photodegradation of MO was analyzed by UV-Vis double beam spectrophotometer (UVD-2960) at its maximum absorption wavelength of 464 nm.
Results and Discussion
Characterization of Nanoparticles
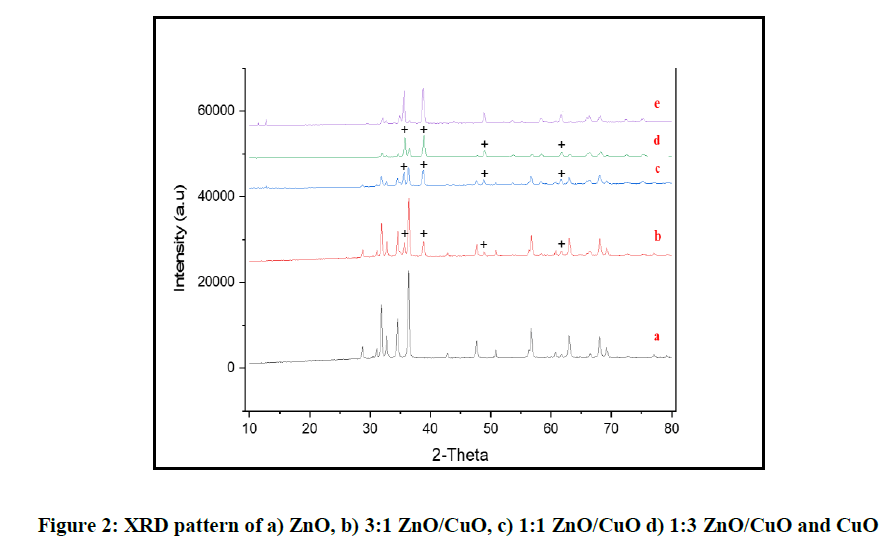
XRD patterns ZnO/CuO composite: XRD patterns of photocatalysts are presented in Figure 2. According to DB Card no. 1-082-0082, the main peaks of pure ZnO were found at 2 θ angles = 31.88 (100), 34.48 (002), 36.37 (101), 47.64 (102), 56.69 (100), 62.93(103), 66.48 (200), 68.05(112), 69.16 (201), and 77.11 (202) symbolized hexagonal wurtzite phase of crystalline ZnO with space group P63 mc, cell constants a=b= 3.2 Å, c= 5.2 Å [11,12]. According to DB Card no. 01-077-7716, the main peaks of pure CuO were found at 2 θ angles = 32.59 (110), 35.61 (-111), 38.74 (111), 48.87 (20˗2), 58.24 (202), 61.66 (˗113), 65.94 (022), 66.35 (31˗1), and 68.13 (113), symbolized monoclinic phase of crystalline CuO (space group C12/c1) a=4.6 Å, b=3.4 Å, c=5.1 Å. Presence of reflection plane (111) of CuO in nanocomposites refers that a part of CuO has been doped with ZnO lattice and thus confirms the formation of ZnO/CuO nanocomposite. The intensity of reflection plane (111) increases as the amount of CuO increase [13,14], no diffraction peaks of other phases appear in XRD patterns of ZnO/CuO nanocomposite which mean the formation of ZnO/CuO nanocomposite without any impurities as confirmed by EDX analysis. The crystal size (D) was measured by Scherrer’s equation [15].

Where D is the crystalline size in Å, λ is the X-ray wavelength (1.54 Å ), β is Full Width at Half Maxima (FWHM) of observed peak, and θ is Bragg’s diffraction angle. The prepared photocatalysts exhibits sharp peaks in XRD patterns and the crystalline size is less than 100 nm which mean nanocrystalline nature of these composites. Dislocation density (δ ), microstrain (ε ) and lattice constants (a,c) were calculated at the most instance peak at reflection plane (101) of ZnO (Table 1). Microstrain values of ZnO/CuO nanocomposites vary with varying molar ratio of the samples while the ratio c/a is found to be 1.732 which conforms the formation of close packed structure, The change in dislocation density, microstrain ( ε ) and lattice constants due to the incorporation of Cu+2 into Zn+2 during the crystallization (Figure 2).
| Photocatalyst | Crystalline size (nm) | Dislocation density ( δ ) | Microstrain ( ε ) | Lattice constants | Ratio c/a |
|
|---|---|---|---|---|---|---|
| c | a | |||||
| ZnO | 42.02 | 0.566 | 0.254 | 6.216 | 3.589 | 1.732 |
| ZnO/CuO(3:1) | 36.86 | 0.736 | 0.2804 | 4.931 | 2.847 | 1.732 |
| ZnO/CuO(1:1) | 32.31 | 0.958 | 0.396 | 4.919 | 2.840 | 1.732 |
| ZnO/CuO(1:3) | 35.30 | 0.803 | 0.2916 | 4.933 | 2.848 | 1.732 |
| CuO | 27.37 | |||||
Table 1: Crystalline size, Dislocation density ( δ ), microstrain ( ε ) and lattice constants (a,c) of the prepared photocatalyst.
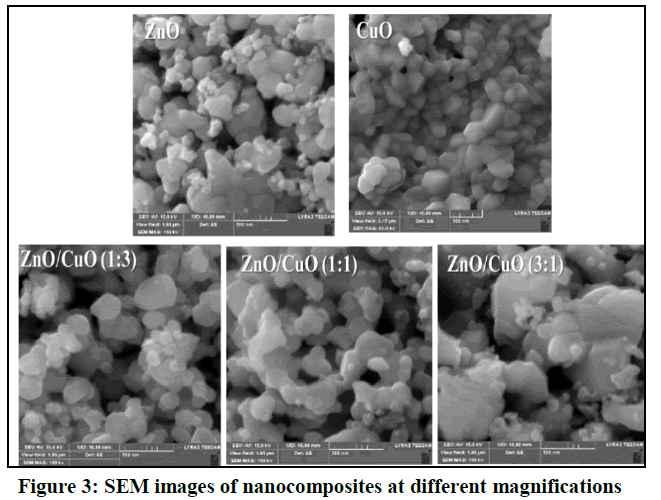
Morphological studies of the ZnO/CuO nanocomposite: The morphology of ZnO, CuO and ZnO/CuO nanocomposite was evaluated using Scanning Electron Microscopy (SEM) (Figure 3). The SEM micrograph of pure ZnO nanoparticles showed homogeneous distribution of spherical morphology with size range from 22-50 nm. The synthesized CuO nanoparticles found to be well defined, spherical identical crystalline structure agglomerated and form a cluster. The ZnO/CuO nanocomposites SEM images showed remarkable morphology change in shape and particle size. This is due to the incorporation of Cu+2 into Zn+2 during the crystallization. Sample ZnO/CuO (3:1) with high ZnO molar ratio Zn 2.86/CuO 4.04 consist of small irregular spherical like shape structure with size from 35-70 nm which is aggregate in larger one. As ZnO molar ratio decrease in sample ZnO/CuO (3:1) which shows nodular spherical like shape in particle size around 32-100 nm. Meanwhile the morphology of sample ZnO/CuO (1:3) shows agglomeration of the nanoparticles with small particle (30-100 nm) appears on the surface.
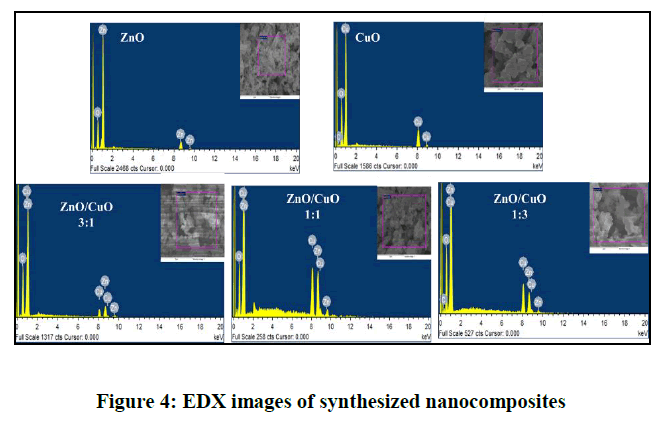
The EDX gave information about the elemental distribution of the nanoparticles and purity of the samples (Figure 4), the EDX of the pure oxides showed two peaks one for oxygen and the second for corresponding metal Zn in ZnO and Cu in CuO respectively. While the EDX of the different molar ratio ZnO/CuO nanocomposites gave peaks indicate the presence of the three elements which support the formation of the ZnO/CuO nanocomposite. It is worth to mention the oxygen peak conforms the presence of zinc and copper in their oxidized form (Table 2). The absence of impurity peak in all the spectra is prove of the purity of the prepared photocatalyst.
| Sample/molar ratio | Weight% (atomic%) | ||
|---|---|---|---|
| Zn | Cu | O | |
| ZnO | 76.78 (55.27) | - | 23.22 (44.75) |
| ZnO/CuO (1:3) | 59.32 (36.19) | 20.17 (12.66) | 20.5 (51.14) |
| ZnO/CuO (1:3) | 41.26 (22.13) | 33.45 (18.46) | 19.79 (43.37) |
| ZnO/CuO (1:3) | 52.69 (34.49) | 30.49 (20.54) | 16.81 (44.97) |
| CuO | - | 71.79 (37.82) | 23.7 (49.59) |
Table 2: Composition analysis of the nanoparticles using EDX
Photocatalytic Degradation of Methyl Orange MO
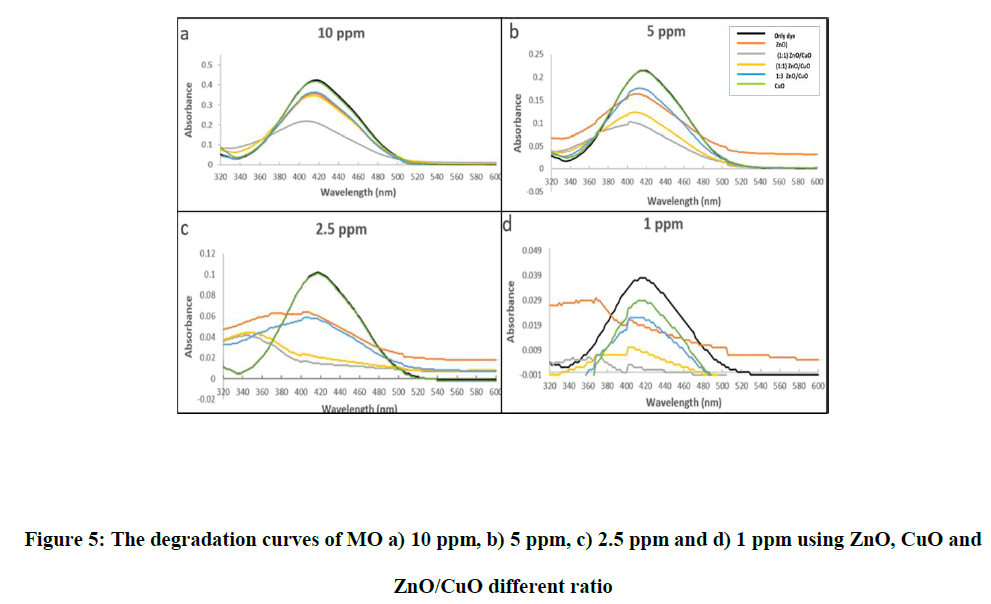
Photocatalytic degradation efficiency of the prepared material was evaluated at different methyl orang concentration (1,2,5,5,10 ppm). The absorption spectrums at 464 nm gradually decreased and achieved the maximum degradation at 180 min. In the present work, 1 ppm of MO at pH 7 with 100 mg of photocatalyst was chosen to assess the photodegradation efficiency.
The degradation of MO (removal efficiency) was calculated by applying the equation

Where %D: percent degradation (degradation efficiency), Ao the initial absorbance of methyl orange at time=0 min and At is the absorbance after time=t min. Generally, the degradation efficiency of ZnO/CuO nanocomposite is higher than that of the corresponding pure oxides and increase with increase ZnO molar ratio in ZnO/CuO nanocomposite and decrease of MO concentration.
The optimum concentration of ZnO/CuO (3:1) nanocomposite which displays the highest photocatalytic activity (100% removal of dye after 180 min) because CuO provides effective charge transfer of narrow bandgap semiconductor of CuO to the wide bandgap of ZnO semiconductor, thus diminish recombination of charge carriers by electrons transfer. While sample ZnO/CuO (1:3) reduces the photocatalytic activity due to the increase in the amount of CuO, which leads to covering the surface and active sites of ZnO, and thus reduces the absorption of UV irradiation (Figure 5).
Kinetic Studies of Photocatalytic Degradation of Methyl Orange MO
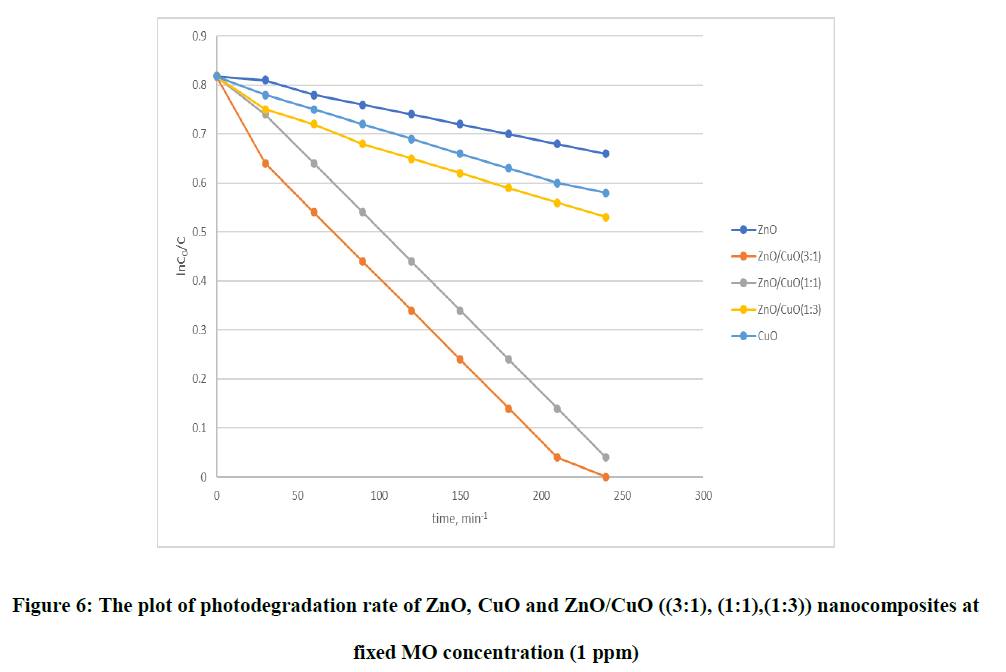
The kinetic behaviour for MO photodegradation fallows pseudo first order kinetic model

Where C0, C represents the initial MO concentration (t=0) and residual concentration (t=t). The higher the degradation rate constant kapvalue means high degradation efficiency for MO dye (Figure 6). These results suggests that the ZnO/CuO photocatalyst can greatly improve the photocatalytic degradation of MO compared to pure ZnO and CuO under light irradiation. The trend of photocatalytic activity of this material follows this order ZnO, CuO, (1:1) ZnO/CuO, (1:3) ZnO/CuO and (3:1) ZnO/CuO for the degradation of methyl orange dye (Table 3).
| Compounds | % Efficiency | Rate constant k(min-1) | Regression constant |
|---|---|---|---|
| ZnO | 27% | 6.79 × 10-4 | 0.996 |
| CuO | 50% | 9.84 × 10-4 | 0.998 |
| ZnO/CuO (1:3) | 100% | 3.28 × 10-3 | 0.988 |
| ZnO/CuO (1:3) | 94.9% | 3.13 × 10-3 | 0.999 |
| ZnO/CuO (1:3) | 66.6% | 3.37 × 10-3 | 0.986 |
Table 3: % efficiency, reaction rate, and regression constant of MO (1 ppm)
Mechanism Photocatalytic Degradation of Methyl Orange
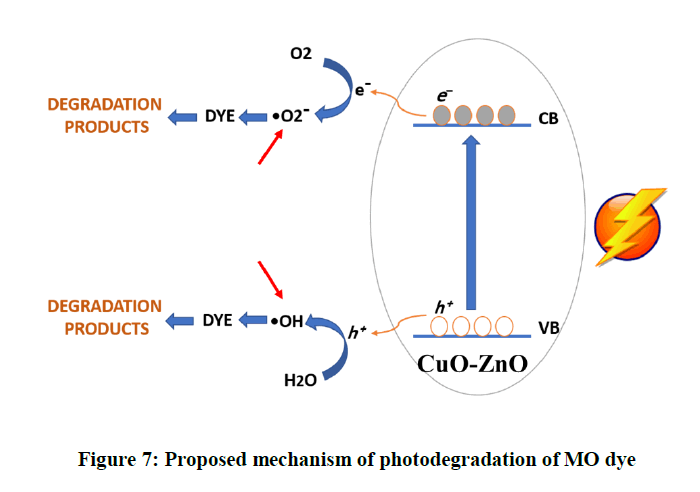
The expected mechanism of photodegradation shown as follows. When directing light on the photocatalyst the ZnO/CuO nanocomposite, electrons of CuO will be excited (1) and immigrate zfrom the valence band into the ZnO conduction band (2) forming the positive holes in both semiconductors. Since ZnO is located lower than CuO, the electrons transferring process to and from the valence and conduction band between them is easy. Meanwhile, produced electrons perhaps interact with O2 and decompose the dye (3 and 6). While the h+ in CuO maybe react with OH¯ or H2O formed H2O2 and OH• (4 and 5). (Figure 7)
CuO-ZnO + ℎν → CuO (e¯) CB + CuO (h+) VB-ZnO……… (1)
Zn (e¯) + O2 → ZnO + O2• ……… (2)
CuO (e¯) VB + O2 →CuO + O2 •¯……… (3)
CuO (h+) VB + OH¯ →CuO + OH• ……… (4)
MO + OH• → products ……… (5)
MO + O2•¯ →products……… (6)
Conclusion
In summary, we have synthesized different molar ratios of binary metal oxides ZnO/CuO for photodegradation of methyl orange under UV irradiation. XRD results explicate the hexagonal wurtzite phase of ZnO and monoclinic phase of CuO. ZnO/CuO (3:1) completely degrades the dye after 180 min of illumination time. Doping of zinc oxide with copper oxide is a good method to fabricate effective photocatalyst enhancing the degradation of wastewater dyes.
References
- Viswanathan B. CCAT. 2018; 7(2):99-121.
- Ohtani B. J. Photochem. Photobiol. 2010; 11(4):157-78.
- Kain M L, Chin W L, Koh S,et al. Water Res. 2016; 88(1):428-448
- Ong CB, Ng LY, Mohammad AW. Renew. Sustain. Energy Rev. 2018; 81:536-51.
- Das S, Srivastava VC. Nanotechnol. Rev.
- Gajendiran J, Rajendran V. Mater. Lett. 2014;116:311-3.
- Li B, Wang Y. Superlattices Microstruct. 2010; 47(5):615-23.
- Susmitha D, Srivastava VC. J. Nano Res. 2016; 35:21-26.
- Gerawork M. Optik,2020; 216:164864.
- Jan T, Azmat S, Mansoor Q, et al. Microb. Pathog. 2019; 134:103579.
[Cross Ref] [Google Schlor] [Pub Med]
- Acedo-Mendoza AG, Infantes-Molina A, Vargas-Hernández D, et al. Mater Sci Semicond Process. 2020; 119:105257.
- Ghule LA, Patil AA, Sapnar KB, et al. Toxicol Environ Chem. 2011; 93(4):623-34.
- Mansournia M, Ghaderi L. J. Alloys Compd. 2017;691:171-7.
- Mukhtar M, Munisa L, Saleh R. MSA.2012; 03:543-551.
- Crank J.414.