Original Articles: 2023 Vol: 15 Issue: 6
Synthesis of Vertical Graphene Nano walls by Plasma Enhanced Chemical Vapor Deposition
Enric Bertran-Serra*, Stefanos Chaitoglou, Roger Amade Rovira, Rogelio Ospina, José Luis Andújar-Bella
Department of Applied Physics, Universitat de Barcelona, Martí i Franques 1, E-08028 Barcelona, Spain
- Corresponding Author:
- Enric Bertran-Serra
Department of Applied Physics, Universitat de Barcelona, Martí i Franques 1, E-08028 Barcelona, Spain
Received: 23-May-2023, Manuscript No. JOCPR-23-100867; Editor assigned: 29-May-2023, PreQC No. JOCPR- 22-100867 (PQ); Reviewed: 12-Jun-2023, QC No. JOCPR-22-100867; Revised: 21-Jun-2023, Manuscript No. JOCPR-22-100867 (R); Published: 29-Jun-2023, DOI:10.37532/0975-7384.2022.15 (5).035.`
Citation: Serra EB, Chaitoglou S, Rovira RA, et al. 2023. Synthesis of Vertical Graphene Nano walls by Plasma Enhanced Chemical Vapor Deposition. J. Chem Pharm. Res., 15:035.
Copyright: © 2023 Serra EB, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Vertical Graphene Nano Walls (GNWs) have garnered significant attention in recent years due to their unique properties, including high specific surface area, excellent electrical conductivity, scalability, compatibility with transition metal compounds, and facile growth on diverse substrates. These distinctive characteristics make GNWs a promising material for various applications, such as energy storage, catalysis, and sensing. Consequently, there is growing anticipation regarding their utilization in commercial graphene-based sensors, electrodes for energystorage systems, and catalytic applications. Among the various methods employed for graphene synthesis, Plasma- Enhanced Chemical Vapour Deposition (PECVD) has emerged as a promising technique for achieving large-scale graphene films on electrodes. During the growth process, the formation of defects at the nano wall edges provides anchoring sites for functionalization with transition metal oxides, carbides, and nitrides, leading to enhanced specificity and efficiency in different applications. Despite substantial progress in optimizing growth conditions to attain micrometer sized graphene nano walls, the outcomes are often obtained through empirical approaches. A comprehensive understanding of the underlying physicochemical mechanisms that govern the formation of Nanostructures remains elusive. Exploring atomic-level phenomena such as nucleation, adsorption of carbon precursors, and surface diffusion of ad atoms is imperative to exert precise control over the growth process. Previous studies have emphasized the dual role of hydrogen in graphene growth, serving as both a co-catalyst and an etchant for the nano walls. However, a thorough comprehension of the intricate growth process is still required.
Keywords
Graphene; Vapour deposition; Transition.
Introduction
In this review paper, our objective is to investigate the PECVD growth of graphene by examining existing theories that elucidate the two-step process involved: nucleation as a graphene film and subsequent growth of vertical nanostructures through the overlapping of growing graphene islands during their coalescence phase. These theoretical frameworks offer valuable insights into the pivotal stages determining nucleation density, distribution of nuclei, and final coverage. Specifically, we aim to explore the temperature effect on the graphene growth ratio and nucleation density across a wide temperature range. By delving into the fundamental principles governing the growth of graphene nanowalls, this review seeks to provide a comprehensive understanding of the PECVD process. The insights gained from this analysis will contribute to the development of optimized growth conditions, enabling precise control over the formation of vertical graphene nanowalls with tailored properties for various applications in energy storage, catalysis, and sensing.
Literatutre Review
In the vast realm of graphene growth experiments, a diverse range of setups and procedures have been employed. One studies by Chaitoglou, et al. [1] an explored graphene nucleation through CVD growth at temperatures ranging from 970 to 1070°C. By examining the effects of temperature on graphene growth ratio and nucleation density, they estimated an activation energy of 3.01 eV. Another earlier investigation by Ando, et al. [2] involved DC arc-discharge evaporation of graphite in a hydrogen-filled vacuum chamber, resulting in carbon deposits on a small cathode. Scanning electron microscopy revealed interlaced petal-like sheets, while transmission electron microscopy identified smaller nanometric petal-like structures as graphite. A more recent study by Lehmann, et al. [3] Focused on Hierarchical Carbon Nanowalls (HCNWs) synthesized using radio frequency plasma-enhanced chemical vapour deposition. This process utilized an inductively coupled plasma source and p-xylene as the carbon precursor. The HCNWs were deposited on various substrates, including silicon wafers coated with Tin for morphological examinations and electrochemical measurements. Micro Wave (MW) reactors operating in TE mode have been widely employed for Vertical Graphene (VG) synthesis. By utilized a 2.45 GHz MW source coupled to a cylindrical quartz tube via a traverse rectangular cavity waveguide [4] By adjusting the waveguide length with a tuner, they created a standing wave withthe strongest electric field in the growth region. However, TE-MW reactors have limitations such as restricted substrate temperature, potential contamination, and limited operation power. To overcome these issues, the TM-MWreactor was introduced, converting the dominant wave from TE mode to TM mode using a cylindrical waveguide. An antenna placed atop the reactor vessel enhanced the electric field on the substrate, enabling better control of substrate temperature.
The use of a dielectric window prevented overheating and allowed higher operating power and pressure grew Carbon Nano Walls (CNWs) through Inductively Coupled Plasma-Enhanced Chemical Vapour Deposition (ICP- CVD) employing a CH4/Ar mixture they utilized n-type Si (100) and SiO2-coated Si (100) substrates with a 50 nmoxide layer [5]. The ICP reactor consisted of a one-turn coil antenna on a quartz window, generating plasma through rf power (13.56 MHz). The Si or SiO2-coated Si substrates were positioned below the quartz window on the substrate holder. In some cases, Ti-catalyzed SiO2-coated Si substrates were utilized to investigate nucleation enhancement. Catalytic nanoparticles were patterned on the SiO2-coated Si substrate using a lift-off method. Growth experiments were conducted at a substrate temperature of 700°C, rf power of 500 W, and total gas pressure of 1.3 Pa, with specific flow rates of CH4 and Ar.
Characterization techniques included scanning electron microscopy, transmissionelectron microscopy, and Raman spectroscopy. Another notable study by Yu, et al. [6] employed a plasma reactor operatingat atmospheric pressure. The setup comprised a quartz tube housing a tungsten needle cathode, a grounded graphite rod anode, and a dc high negative voltage supply. Argon served as the plasma gas, and substrates such as silicon wafers, stainless steel plates, and copper plates were mounted on the graphite rod. The growth process involved heating the substrate to 700°C in an Ar/H2 flow, followed by switching to an Ar/ethanol flow to initiate plasma generation.
The plasma power reached 2.9 W, and the process lasted for 15 minutes. Subsequently, the as-grown CNWs underwent thermal annealing at 900°C. VGNWs were grown on flexible stainless steel substrates suitable for electrochemical systems such as batteries, supercapacitors, and catalysts [7] graphene nanowalls has mainlyfocused on multi-layered graphene. This study aims to investigate the effect of temperature on the number of atomic layers in VGNWs. The experimental section details the growth conditions and the methodology employed to analyse the structural and morphological characteristics of the VGNWs. The authors discuss the use of ICP-CVD with methane as the carbon precursor.
They provide insights into the plasma conditions, gas flow, and pressure, which arecrucial parameters for modulating the growth process. The advantages of ICP-CVD over other techniques, such as the absence of catalysts and the higher electron density of the plasma, are highlighted. Overall, the methodologies employed for graphene growth and analysis have exhibited significant diversity. Different techniques, such as CVD growth, DC arc discharge evaporation, plasma-enhanced chemical vapor deposition, and microwave reactors, have been utilized with varying parameters, including temperature, pressure, gas composition, and substrate materials. The characterization techniques have encompassed SEM, TEM, Raman spectroscopy, XPS, and sensor current measurements. These Diverse methodologies have contributed to the understanding of graphene growth and the synthesis of unique carbon nanostructures like carbon nanowalls.
Discussion
Among the most interesting achievements we have 3D graphene networks [8]. The fabrication process and properties of 3D graphene networks involves pressing commercially available Ni foam, cleaning it, and coating it with graphene using a chemical vapour deposition method. The resulting 3D graphene network exhibits flexibility, mechanical strength, and high electrical conductivity. The pressed Ni foam prevents the network from collapsing and cracking during bending. The graphene networks are further modified by electrodeposition of MnO2, which results in a uniform coating over the entire surface. The graphene/MnO2 composite demonstrates good capacitance and electrochemical performance, making it suitable for flexible electrode applications. Recent research on vertical graphene is a Nano carbon thin-film material with unique 3D hierarchical structures [9]. Unlike planar growth graphene, vertical graphene has abundant out-exposing edges, high porosity, Nano passages, and a large surfacearea, making it suitable for applications in electrochemistry, bioelectronics, and flexible electronics. The challenges in studying vertical graphene include standardizing nomenclature, characterizing its complex structure, understanding its growth mechanism, and integrating small-sample processing into mass production. Various techniques are being explored, such as using solidstate carbon sources and applying magnetic fields.
Vertical graphene shows promise for commercial applications in areas like flexible electronics, micro energy conversion, electro catalysis, bio sensing, and wearable devices, surpassing conventional graphene and carbon nanotube materials. Water splitting through electro catalysis is another promising method for producing clean and renewable fuel, molecular Hydrogen (H2). Researchers are seeking cost-effective and efficient electrocatalysts, and carbon-based catalysts, particularly graphene, have shown potential. Recently, manipulating the electronic structure of graphene through surface defect sites and functional-group modifications has been found to enhance its electro catalytic activity. In a new study, researchers developed three-dimensional graphene networks with abundant sharp edge sites by depositing vertical graphene sheets on SiOx nanowire networks [10]. The resulting 3D graphene networks exhibited excellent electro catalytic activity for Hydrogen Evolution Reaction (HER), making them highly active and stable metal-free and dopant-free HER electrocatalysts. Also, the study of electrochemical activity of Carbon Nanowalls (CNWs) was examined through the morphology and nanostructure of CNWs synthesized by PECVD [3]. The height of the CNWs increased with deposition time, and they consisted of interconnected vertically aligned carbon sheets. The nanostructure was also characterized using Raman spectroscopy, which revealed defects in the graphitic lattice. X-Ray Photoelectron Spectroscopy (XPS) confirmed the presence of SP2 hybridized carbon and the absence of heteroatom doping. The electrochemically active surface area of the CNWs increased with film thickness and deposition time. However, the peak potential difference in cyclic voltammetry measurements was Larger than expected, possibly due to surface contamination. Overall, the study provides insights into the morphology and electrochemical properties of hCNWs synthesized by PECVD. The study on CNWs grown using ICP-CVD with a mixture of CH4 and Ar accounts for the control of nucleation as a means to improve the characteristics of CNWs [5]. Thus, the growth rate of CNWs was measured, and the nucleation and early growth stages were investigated. The effect of substrate materials, such as Ti-coated Si and Ti-catalyzed SiO2, on CNW growth and nucleation was examined. Scanning Electron Microscopy (SEM) images show vertically grown two-dimensional carbon sheets with a smooth surface, forming a maze-like nanostructure. Transmission Electron Microscopy (TEM) confirms the graphitized structure of the CNWs with a spacing of approximately 0.34 nm between graphene layers. Raman spectroscopy indicates the presence of a graphitized structure.
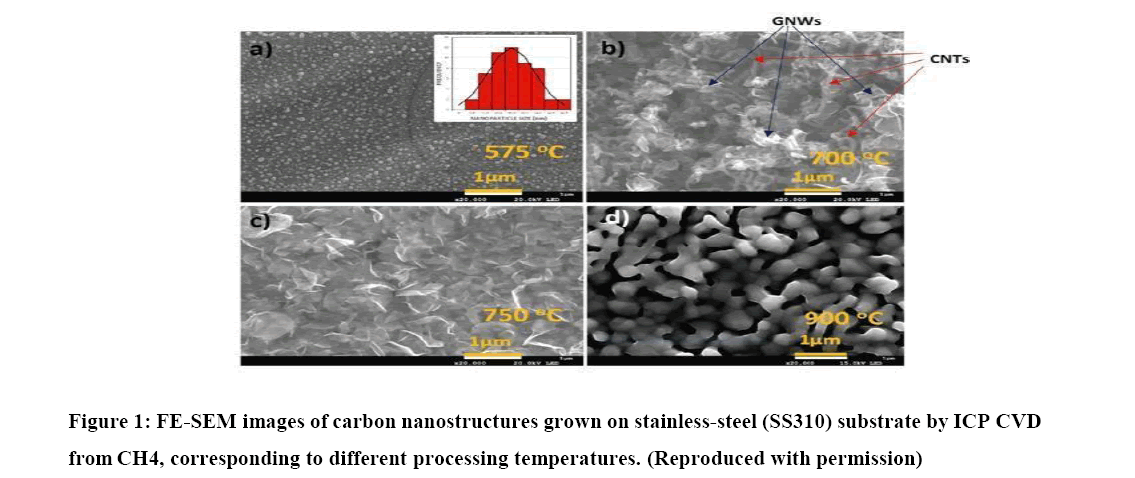
Another fundamental study investigates the effect of growth temperature on carbon nanostructures through Raman spectroscopy and electron microscopy [7]. Carbon nanostructures were grown on SS310 substrates at different temperatures using a remote plasma process. The results show that the growth temperature influences the structure and morphology of the carbon nanostructures. Capacitively-Coupled Plasma (CCP) promotes the growth of Carbon Nano Tubes (CNT), while Inductively-CoupledPlasma (ICP) favors the growth of Vertical Graphene Nano Walls (VGNWs).
The mass deposition rate and Raman spectroscopy were used to analyse the carbon nanostructures, confirming the presence of graphene nanowalls and carbon nanotubes at different growth temperatures. The study provides valuable insights into the growth mechanisms of carbon nanostructures. Finally, the study conducted by Yu, et al. [6] presents the results and discussion on the synthesis of Carbon Nano Wires (CNWs) using an atmospheric pressure Direct Current Plasma-Enhanced Chemical Vapour Deposition (DC PECVD) system, that extend the technologies to facilitate the fabrication process.
The morphology of the as grown CNWs was analyzed through Scanning Electron Microscopy (SEM) images, showing uniform distribution on the substrate with dimensions ranging from 200 × 200 nm² to 1 × 1 μm2.
Raman spectroscopy revealed the presence of graphitic carbon in the CNWs, while X-Ray Photoelectron Spectroscopy (XPS) indicated a reduction in oxygen functional groups after thermal annealing. Transmission Electron Microscopy (TEM) confirmed images the few- layered structure and crystallinity of the CNWs (Figure 1).
Conclusion
CNWs can be synthesized via Plasma Enhanced Chemical Vapour Deposition (PECVD) without the use of a catalyst. The height of CNWs exhibits a linear increase with the growth time. Moreover, CNWs can be grown on different substrates such as Si, SiO2, and Ti nanoparticle-coated SiO2, stainless-steel, and Ni, among others, withoutthe requirement of a catalyst. The growth of planar nanographenes on Ti nanoparticles indicates the potential for areaselective growth, opening avenues for device applications. The electrochemical properties of CNWs, including their electron transfer activity and catalytic performance towards the oxygen reduction reaction, position them as promising candidates for high-performance electrodes. Freestanding flexible 3D graphene/MnO2 composite networks exhibit exceptional electrochemical performance, making them well suited for flexible supercapacitors. These networks, prepared from pressed Ni foam, demonstrate superior mechanical strength and flexibility. A substantial mass of MnO2 can be uniformly coated onto the entire network skeleton through electrodeposition. The remarkable electrochemical behaviour of this composite material can be attributed to multiple factors. The highly Conductive and porous structure of the 3D graphene networks allows efficient electron transfer and electrolyte ion (Na+) access, maximizing charge storage. The strong contact between graphene and MnO2 ensures low contact resistance and robust adhesion, enabling the material to withstand repeated bending deformations.
Additionally, the large specific surface area of the 3D graphene networks provides ample active surface area for effective utilization of the pseudo capacitive MnO2 material.SS310 stainless steel emerges as a suitable substrate for carbon nanostructures, although the growth temperature significantly impacts the resulting nanostructures. Curly carbon nanotubes and vertically grown graphene nanowalls are the prevalent carbon nanostructures obtained through PECVD on SS310 substrates. Raman spectroscopy analysis reveals that graphene nanowalls grown at temperatures ranging from 675°C to 775°C consist of at least two monatomic layers, with defects primarily located at the edges. The presence of graphene nanowalls enhances the active surface area of electrodes by a factor of nine compared to planar substrates. However, temperatures exceeding 800°C lead to surface changes in the SS310 substrate, hindering the growth of carbon nanostructures. Inductively coupled plasma CVD offers a cost-effective approach for synthesizing carbon nanostructures on diverse substrates, including Si, stainless steel, and Cu. These nanostructures hold promise for various applications such as energy storage systems, catalytic systems, and gas sensors, due to their unique properties and compatibility with different materials.
Acknowledgment
The authors acknowledge financial support from project PID2020-116612RB-C32 funded by MCIN/AEI/ 10.13039/501100011033. The ENPHOCAMAT group acknowledges support from the AGAUR of Generalitat de Catalunya, Project No. 2021SGR0936. S.C. acknowledges support from the postdoctoral fellowships programme Beatriu de Pinós, funded by the Secretary of Universities and Research (Government of Catalonia) and by the Horizon 2020 programme of research and innovation of the European Union under the Marie Sklodowska-Curie grant agreement No 801370 (H2020 MSCACOFUND-2017).
References
- Chaitoglou S, Bertran E. J Mater Sci. 2017; 52 (13): 8348–8356.
- AndoY, Zhao X, Ohkohchi M. Carbon. 1997; 35 (1):153–158.
- Lehmann K, Yurchenko O, Melke J, et al. Electrochimica Acta. 2018; 269(1):657–667.
- Bo Z, Yang Y, Chen J, et al. Nanoscale. 2013; 5 (12): 5180.
- Hiramatsu M, Nihashi Y, Kondo H, et al. Jpn J Appl Phys. 2013; 52 (1S): 01AK05.
- Yu K, Bo Z, Lu G, et al. Nanoscale Res Lett. 2011; 6 (1):202-205.
- Bertran-Serra, E, Musheghyan-Avetisyan A, Chaitoglou S, et al. App Surface Sci. 2023; 610(1):155530.
- He Y, Chen W, Li X, et al. ACS Nano. 2013; 7 (1): 174–182.
- Zheng W, Zhao X, Fu W, et al. ACS Appl Mater Interfaces. 2021; 13 (8): 9561–9579.
- Wang H, Li XB, Gao L, et al. Angew Chem Int. Ed. 2018; 57 (1):192–197.