Original Articles: 2025 Vol: 17 Issue: 1
Synthesis of Dithiocarbamates via Baylis-Hillman Adducts and their Theoretical Investigation with the Aid of Different Analytical Tools: A Review
Suman Gill, Vipin Kumar, Ashish Kumar Tewari*
Department of Chemistry, Institute of Science, Banaras Hindu University, Varanasi, India
- Corresponding Author:
- Ashish Kumar Tewari
Department of Chemistry
Institute of Science, Banaras Hindu University
Varanasi,
India
Received: 18-May-2024, Manuscript No. JOCPR-24-136414; Editor assigned: 20-May-2024, PreQC No. JOCPR-24- 136414 (PQ); Reviewed: 03-May-2024, QC No. JOCPR-24-136414; Revised: 03-Jan-2025, Manuscript No. JOCPR- 24-136414 (R); Published: 10-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).235
Citation: Gill S, et al. 2025. Synthesis of Dithiocarbamates via Baylis-Hillman Adducts and their Theoretical Investigation with the Aid of Different Analytical Tools: A Review. J. Chem Pharm. Res., 17:235.
Copyright: © 2025 Gill S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Dithiocarbamates (DTCs), known for their diversity among organosulphur compounds, have sparked great scientific interest due to their intriguing characteristics and prospective applications. This review presents a comprehensive overview of DTC synthesis and aims to highlight the recent advancements. This article mainly focuses on the synthesis of DTCs from Baylis-Hillman adducts. The crystallographic study helps in interpreting intramolecular interactions in the solid state thus broadening their synthetic applicability. Hirshfeld surface analysis focuses on intermolecular interactions in DTCs’ crystal packing. Density Functional Theory (DFT) study contributes to the understanding of electronic properties and provides a theoretical framework for predicting their reactivity and stability. Molecular docking interprets the interactions of diverse biological targets with DTCs and provides significant insights into potential therapeutic uses.
Keywords
Dithiocarbamates; Crystallographic study; Hirshfeld surface analysis; Density functional theory; Molecular docking study
Introduction
It is widely recognized that naturally occurring substances, pharmaceutically active compounds and organic materials contain carbon-sulfur bonds [1]. Among the many organosulfur compounds, Dithiocarbamates (DTCs) have been considered to be the simplest. These compounds feature core carbon connected to two sulfur atoms and one nitrogen atom, which is further connected to two alkyl or aryl groups. Because of their many uses and adaptable characteristics, DTCs have attracted a lot of study interest [2]. DTCs often serve as ligands in coordination chemistry and metal extraction procedures due to their widely regarded chelating properties [3]. Additionally, their strong biological properties make them useful in a variety of fields [4], including catalysis [5], medicine [6], and agriculture [7]. Current studies have focused on synthesizing novel DTC derivatives with tailored functionalities, exploring their potential as antioxidants and anticancer agents. Furthermore, studies on their toxicity profiles and effects on the environment are still essential to understanding their significance in biological and industrial contexts.
As a result, understanding the molecular mechanisms of DTCs and developing effective synthetic approaches have the potential to advance a variety of research endeavors [8]. DTCs are utilized as precursors for the synthesis of diverse materials including polymers, nanoparticles and thin films [9], owing to their ability to act as sulfur sources [10]. In addition, they are utilized in industries as corrosion inhibitors, facilitating the creation of stronger and more effective materials [11].
In this mini-review, we are compiling the synthetic methods of DTCs from Michael-addition. This review will help connect the voids by presenting a thorough overview of the synthetic methods, characteristics, and potential applications of DTCs. This review is intended to be a useful tool for researchers, encouraging further exploration in this field.
Literature Review
Synthesis of dithiocarbamates
The synthesis of DTCs encompasses diverse strategies. Conventional methods, such as the reaction of secondary amines with carbon disulfide in the presence of a base, have been utilized for a long time due to their simplicity and effectiveness [12]. The synthesis of DTCs has seen significant advancements, particularly in the areas of transition metal-catalyzed reactions [13], photochemical methods [14] and microwave-assisted synthesis [15]. DTC derivatives can also be produced via several different methods, including the reaction of amines with Michael acceptors, organic thiocyanates, allylacetate, or tosyl hydrazone, as well as thiophosgene or isothiocyanates (costly and hazardous chemicals) [16]. In addition, their derivatives can be prepared from Baylis-Hillman adducts.
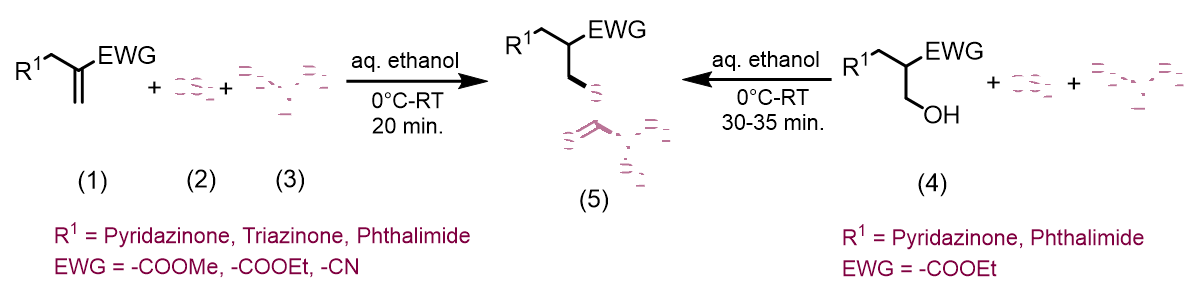
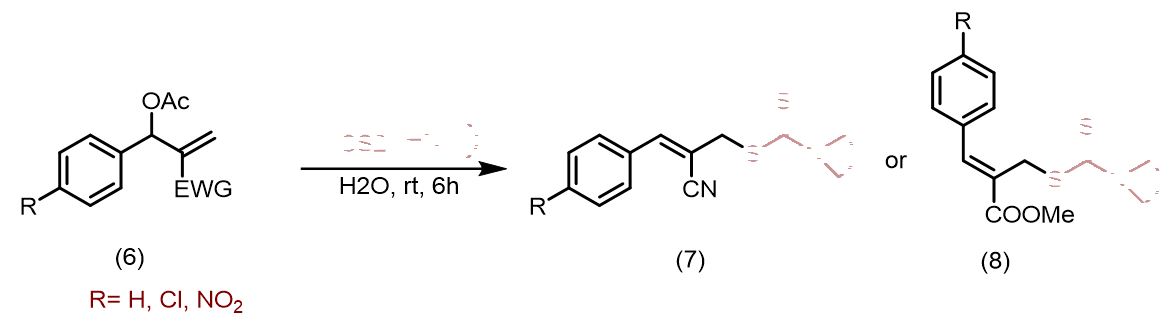
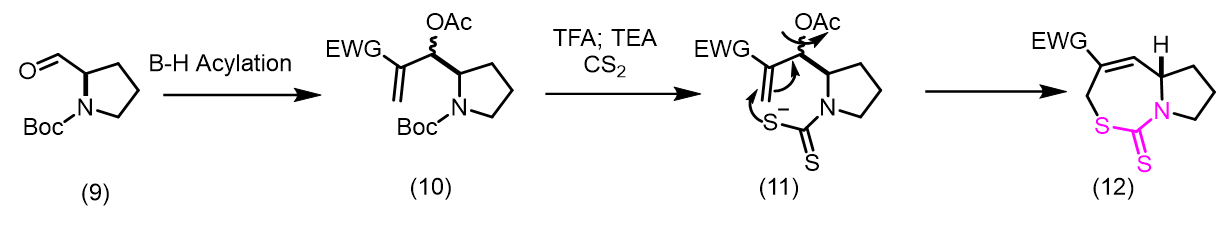
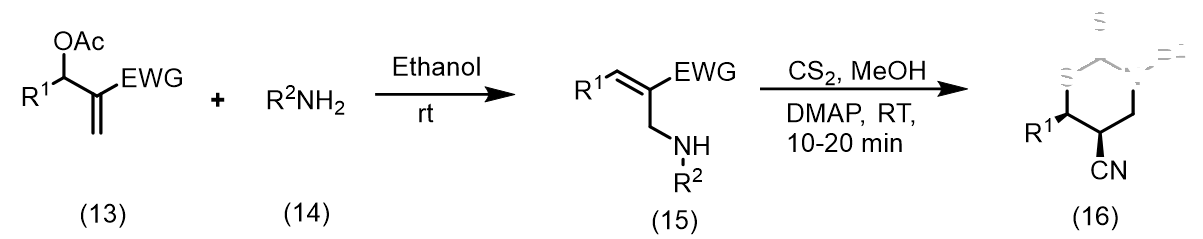
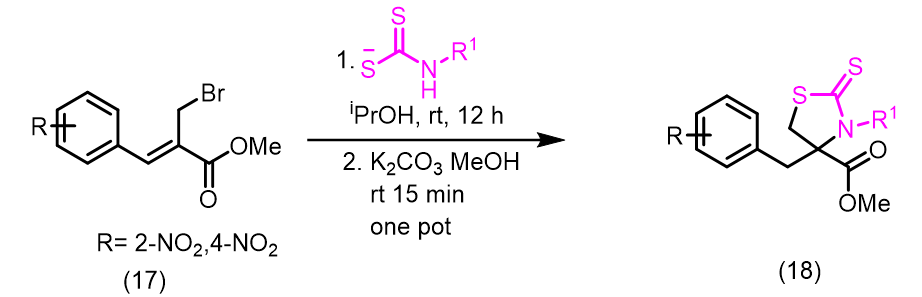
Tewari et al., synthesized new organic dithiocarbamates in aqueous ethanol using a one-pot multicomponent reaction involving CS2, Baylis-Hillman adducts/alcohols, and secondary amines at room temperature. This method is simple, economical and environmentally friendly due to its rapid reaction times and excellent yield (Figure 1). Yadav et al., reported a simple, highly stereoselective, and straightforward synthesis of [E]- and [Z]-allyl dithiocarbamates and through catalyst-free one-pot three-component coupling reactions of amine and carbon disulfide in water under a mild, green process with high yields [16]. The mechanism of the reaction involves dithiocarbamate anions nucleophilically displacing Baylis-Hillman acetates (SN2') (Figure 2). Clive et al., reported N-protected β to the hydroxyl group into novel seven-membered heterocycles that include both sulfur and nitrogen [17]. This reaction is mediated by O-acylation (EtOCOCl or AcCl), N-deprotection (CF3CO2H), and reaction with CS2. The ring closures take place via intramolecular conjugate displacement via intermediate (Figure 3) [18]. Basavaiah et al., reported a method of formation of cyclic dithiocarbamates from ambiphilic molecules (allylamines 15) [19], which are easily accessible from the Baylis-Hillman acetates via the reaction with CS2 using DMAP in presence of methanol (Figure 4) (Figures 1-5). Batra et al., made a report on the synthesis of allyl dithiocarbamates which can be easily obtained from Baylis-Hillman bromides in an alcoholic medium, which further gives substituted 2- thioxothiazolidine-4-alkanoates via intramolecular cyclization in the presence of a mild base (Figure 5) [20].
Figure 1: This method is simple, economical and environmentally friendly due to its rapid reaction times and excellent yield.
Figure 2: The mechanism of the reaction involves dithiocarbamate anions nucleophilically displacing Baylis-Hillman acetates.
Figure 3: The ring closures take place via intramolecular conjugate displacement via intermediate.
Figure 4: The Baylis-Hillman acetates via the reaction with CS2 using DMAP in presence of methanol.
Figure 5: The synthesis of allyl dithiocarbamates which can be easily obtained from Baylis-Hillman bromides.
Discussion
Intramolecular study
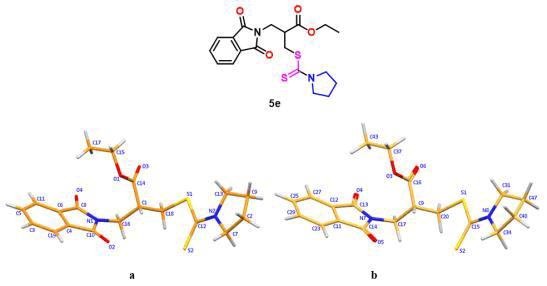
The crystal structure of compounds is the experimental verification in solid state. Tewari et al., discussed the geometry of the DTC molecule which is stabilized by different intramolecular contact. Quantitatively four C–H⋅⋅⋅O, two C–H⋅⋅⋅S, and nine C–H⋅⋅⋅π interactions were observed in compound 5e. The ORTEP diagram of compound 5e is depicted in Figure 6. To confirm the exact conformer of the compound, theoretical calculations have been performed. It has been seen that in experimental measurements, all the C–H, C–C, C–N, C-S, and C–O bonds were shorter than those found in the DFT calculation. This is due to a crystal field and intermolecular interactions holding molecules together in the solid state, accounting for the variations in bond parameters between estimated and observed values. DTCs are the most popular molecules that work as flexidentate ligands, connectivity of sulfur atoms to the metal center as monodentate, as well as bidentate.
Figure 6: a) Ortep diagram of compound 5e, b) Optimized structure of compound 5e.
Hirshfeld surface analysis
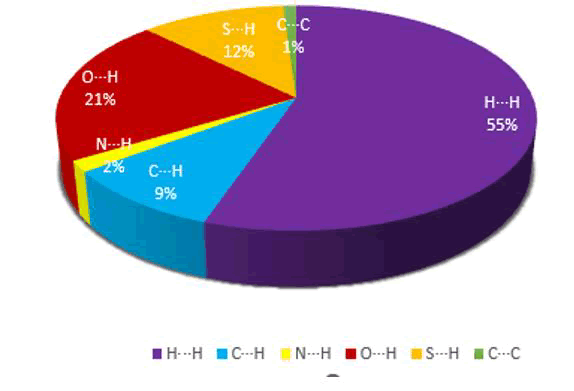
Molecular Hirshfeld surfaces in crystal structures were created using electron distribution calculations based on spherical atom electron densities. The Hirshfeld surface is remarkable due to its crystal structure and spherical atomic densities. Using the normalized contact distance (dnorm), which takes into account both the de and di radii, as well as the vdW radii of the atoms. Intermolecular interactions in the crystal are summarised by the combination of de and di in the form of a two-dimensional (2D) fingerprint plot. Fingerprint plots provide a thorough analysis of intermolecular interactions inside crystals, making them ideal for studying changes in crystal packing in molecular systems. Various studies were evaluated for the Hirshfield surface analysis of DTCs and different types of intermolecular interactions are observed in DTCs containing metal complexes or molecules. Usually, DTCs show C-H…S and S…S intermolecular interactions which participate in the crystal packing of molecules. Bulkiness of carbon fragments attached to the metal center, resulting in a decreasing percentage of C-H…S interactions. The percentage contributions of C-H, N-H, O-H, C-C, S-H, and H-H of compound 5e are depicted in the Pi chart Figure 7.
Figure 7: The percentage contributions of C-H, N-H, O-H, C-C, S-H, and H-H.
Density functional theory
The molecules' geometry, electronic and thermodynamic parameters were obtained from Density Functional Theory (DFT) calculations. The structure optimization of DTCs has been done DFT approach with the functional B3LYP and basis set 6-31g(d,p) but geometry optimization of metal complexes has been done with B3LYP and basis set 6–311G(d,p) {C, H, N, O, S}/ Lanl2DZ. The Lanl2DZ basis set includes the pseudopotential of core electrons in metal atoms. In this review, DFT calculation focuses on the structure of DTCs and their complex models determined by the single crystal X-ray diffraction that were used as starting sets. The Bond lengths and bond angles (Bite angle, S-Metal-S) have small differences between their theoretical and experimental values because experimental parameters originate in intermolecular interactions whereas theoretical parameters are observed in the gaseous state. In a gaseous state intermolecular contact with neighboring molecules are absent. Tewari et al., calculate the electronic transition from HOMO to LUMO and compare the geometry of the DTCs molecules experimental and theoretical parameters. DTC works as a ligand in metal complexes thereby donating and accepting the tendency of the metal in the metal complex of DTCs which leads to the effect of stability of molecules.
Computational ligand-receptor interaction study
Molecular docking analysis is an important computational technique for drug discovery. It is a computational method for detecting molecular interactions. It forecasts the optimal binding of a small molecule (ligand), with a target macromolecule, generally a protein, to discover potential interactions and evaluate pharmaceutical effects. This approach discovers the conformations, generates and evaluates possible ligand-receptor complexes, and predicts the binding modes and affinity. It helps in identifying potential drugs and their interactions with target proteins, which is important in designing and improving novel medications.
DTCs show a wide range of biological properties and thus expand great interest in medicinal chemistry. Recent studies show that DTCs possess the potential to be very effective enzyme inhibitors, making them fascinating choice for future pharmaceutical development. In this regard, DTCs’ molecular docking studies can offer valuable insights into their mode of action and potential therapeutic applications and thus, help in predicting and developing more effective therapeutic agents. Through the docking of drugs, the observed intended activity is obtained by analysing interactions between substances and binding pocket residues of target proteins. Several studies have proven that DTC compounds, because of their ability to enter intracellular spaces, can inhibit oxidative system enzymes like as carbonic anhydrase and superoxide dismutase in a variety of protozoa. It binds to the metal centers of these enzymes to chelate metals. Because of this, when the parasite is living inside the host, it is incapable of repairing oxidative damage.
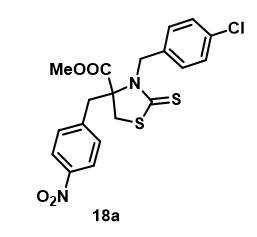
Batra et al., synthesized various substituted 2-thioxothiazolidine-4-alkanoates and were screened for antihyperglycemic activity, one of the analogs (18a) was found to exhibit a significant antihyperglycemic effect by inhibiting the PPARγ at the transcriptional level. This finding was further supported by molecular docking studies (Figure 8).
Figure 8: Structures of 2-thioxothiazolidine-4-alkanoates.
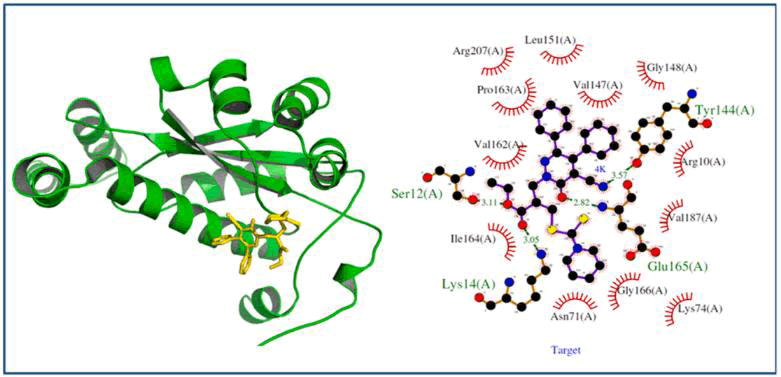
Using molecular docking analysis, Tewari et al., reported several DTCs and evaluated the possible interactions and binding mode to E. coli nitroreductase enzyme. One of their analogous 5a, highly bonded with a binding affinity of -9.4 kcal/mol, forms four hydrogen bonds with Lys14, Ser12, Glu165, and Tyr144. After that, compound 5b has binding affinity of -9.2 kcal/mol. In addition to that, it shows a variety of non-bonded interactions along with hydrogen bond interactions with several residues in the active site. In the same manner, compounds 5c and 5d showed binding affinities of -9.1 kcal/mol and -9.0 kcal/mol respectively (Figure 9).
Figure 9: A variety of non-bonded interactions along with hydrogen bond interactions with several residues in the active site.
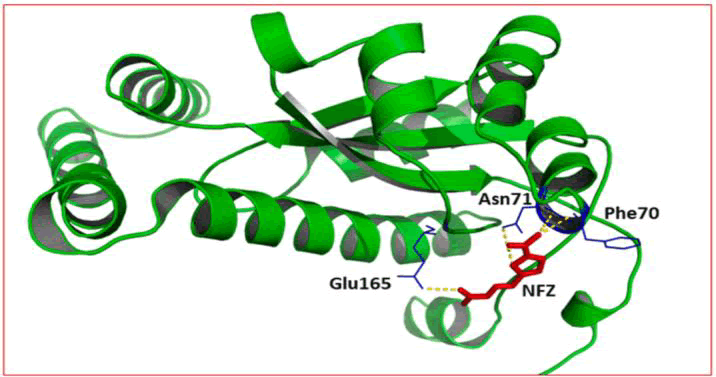
With the target protein's PDB id of 1YKI, Tewari et al. docked their synthetic compounds. They use their crystal structure to bind with Antibiotics Nitrofurazone (NFZ), an antibacterial agent. Along with forming hydrogen bonds with some residues, the ligand NFZ (control) in the binding pocket interacts hydrophobically with other E. coli nitroreductase enzyme residues. Compound (5a) with its remarkable binding affinity forms four hydrogen bonds and shows several non-bonded interactions with other residues. These residues play a crucial role in shaping pocket regions within the target protein. The depiction in Figure 10 portrays the nitroreductase enzyme in conjugation with the NFZ drug and Figure 11 illustrates the complex formed when the leading chemical is docked with the target enzyme.
Figure 10: Nitroreductase enzyme (1YKI): Interactions between protein (green cartoon model) and bound NFZ (red stick model). Pocket-forming residues and hydrogen bonds are shown by blue lines and yellow dotted lines respectively.
Figure 11: Nitroreductase-5a docked complex: The protein is shown in the green cartoon model and the 5a compound is in the yellow stick model inside the pocket. The ligplot shows a hydrogen bond in the green line while hydrophobic bonds are shown (brick-red) spoked arcs.
Conclusion
This review covers an extensive study of DTCs over an assortment of research, encompassing synthesis, inter and intramolecular studies, and biological interactions. The crystallographic study interprets the stability using different intramolecular contacts and flexidentate ligand behaviour. C-H…S and S…S intermolecular interactions are described via Hirshfeld Surface Analysis because of the presence of sulfur atoms in the structure of dithiocarbamates. Molecular docking of DTCs revealed their interactions with protein active sites and hence represented potent drug candidates.
References
- Liu X, et al. Chem Soc Rev. 2015;44:291-314.
[Crossref] [Google Scholar] [PubMed]
- Ahmed AJ. Asian J Chem. 2018;30:2595-2602.
- Onwudiwe DC, et al. Int J Mol Sci. 2022;23:1317.
[Crossref] [Google Scholar] [PubMed]
- Elango KP, et al. Polyhedron. 2015;93:8-16.
- Pang H, et al. Int J Mol Med. 2007;19:809-816.
[Google Scholar] [PubMed]
- Eng G, et al. Appl Organomet Chem. 2003;17:218-225.
- Campanale C, et al. Toxics. 2023;11:851.
[Crossref] [Google Scholar] [PubMed]
- Kanchi S, et al. Arab J Chem. 2014;7:11-25.
- Abd-El-Nabey BA, et al. Phys Chem. 2018;8(1).
- Awang N, et al. Molecules. 2023;28:5841.
[Crossref] [Google Scholar] [PubMed]
- Chen F, et al. J Flow Chem. 2023;13:1-8.
- Shankaraiah N, et al. Green Chem. 2022;24:1259-1269.
- Pourshojaei Y, et al. Res Chem Intermed. 2018;44:1295-1304.
- Yadav LD, et al. Tetrahedron Lett. 2009;50:1335-1339.
- Clive DL, et al. J Org Chem. 2010;75:7014-7017.
[Crossref] [Google Scholar] [PubMed]
- Basavaiah D, et al. Tetrahedron. 2015;71:4659-4664.
- Batra S, et al. Tetrahedron. 2014;70:6841-6850.
- Thirumaran S, et al. J Mol Struct. 2017;1148:547-556.
- Isab AA, et al. J Mol Struct. 2020;1218:128486.
- Ajayi TJ, et al. J Mol Struct. 2019;1197:308-317.