Original Articles: 2024 Vol: 16 Issue: 1
Synthesis, Characterisation and Biological Evaluation of Pyridazine Derivatives
Rudresh HM*, Monica Arora, Slevakumar Balaraman, Datendranath Tripathi
Department of Pharmaceutical Chemistry, Al-Ameen College of Pharmacy, Bangalore, India
- Corresponding Author:
- Rudresh HM
Department of Pharmaceutical Chemistry, Al-Ameen College of Pharmacy, Bangalore, India
Received: 05-Jul-2023, Manuscript No. JOCPR-23-104850; Editor assigned:07-Jul-2023, PreQC No. JOCPR-23-104850 (PQ); Reviewed: 21-Jul-2023, QC No. JOCPR-23-104850; Revised: 27-Dec-2023, Manuscript No. JOCPR-23-104850 (R); Published: 03-Jan-2024, DOI:10.37532/0975-7384.2024.16(01).074.
Citation:Rudresh HM, et al. 2023. Synthesis, Characterisation and Biological Evaluation of Pyridazine Derivatives.
J. Chem Pharm. Res., 16:074.
Copyright: © 2024 Rudresh HM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
A series of 5-substituted(2,3-d)pyridazin-8(7H)-one derivative was synthesized from pyridine dicarboxylic acid, Characterized and evaluated for anticonvulsant activity by Maximal Electroshock (MES) and the subcutaneous-PTZ method using 25 mg/kg of each compound against standard drugs Phenytoin 25 mg/kg and diazepam 4 mg/kg respectively. Among the synthesized derivatives, compounds 5a (100%), and 5b (100%) showed potential activity by the complete abolition of the hindlimb extensor in the MES method, and compounds 5g (72.2%) and 5a (57.4%) showed significant protection against the subcutaneous-PTZ method. Synthesized compounds 5b, 5c, and 5e also exhibited good anti-inflammatory activity against Indomethacin.
Keywords
Pyridazines, Docking studies, In vivo Anti-convulsant activity, In vitro Anti-inflammatory activity, hindlimb extensor
Introduction
A wide number of heterocyclic compounds are active as medicinal compounds and they are also present in a large number of biomolecules which makes them better lead molecules for drug discovery. Pyridazines are compounds containing two adjacent nitrogen heteroatoms which are also believed for their pharmacological activity. Pyridazine derivatives are known for their diversified pharmacological action as they exhibit activities such as anti-inflammatory, antimicrobial, anti-hypertensive, antidiabetic, antioxidant, anticancer, anti-tubercular, and antipsychotic. A large number of studies have shown that pyridazine derivatives are active in the protection of epileptic attacks. Minaprine, Pipofezine (antidepressant), Imazodan (inotropic), Levosimendan (vasodilatory activity), Pimpbendan (anti-CHF), Zardaverine (selective inhibitor of PDE III and PDE IV isozymes), Zopolrestat (neuropathy, and cataract formation), Emorfazone (analgesic and anti-inflammatory) are the compounds with Pyridazine ring moiety which were utilized in the treatment of various clinical diseases and disorders [1].
Epilepsy or seizures are characterized by a sudden, excessive neuronal discharge that is manifested as brief episodes of disturbance of consciousness, with or without characteristic body movements. Based on the electroencephalographic patterns, seizures are majorly classified into Partial (local, focal) seizures and Generalized (convulsive or no convulsive) type seizures. In the systemic and meta-analysis by KM Fiest et al, the pooled incidence rate of epilepsy was found to be 61.4 per 100000 person-years (95% CI: 50.7-74.4) [2].
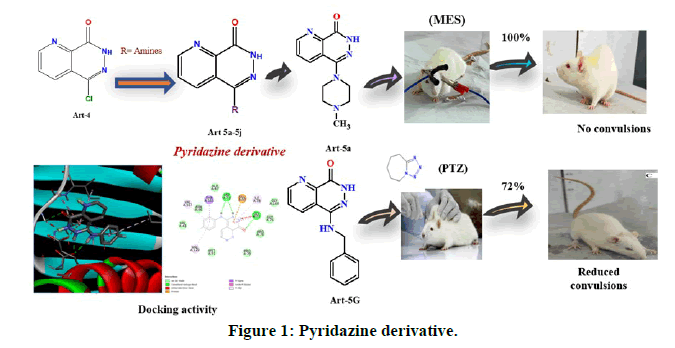
Antiepileptic drugs are used for decades including the second and third generations in the recent times, but they are effective in diminishing the symptoms of epilepsy which fails to prevent epileptogenesis. Older generation drugs have many side effects, whereas multiple-drug therapy is also reported to cause toxicity due to drug-drug interactions. The current work was designed to synthesize new antiepileptic drugs with benefits in terms of tolerability, fewer drug interactions, and simpler pharmacokinetics [3]. The molecular level interaction of synthesized compounds with respective proteins was studied (Figure 1).
RESULTS AND DISCUSSION
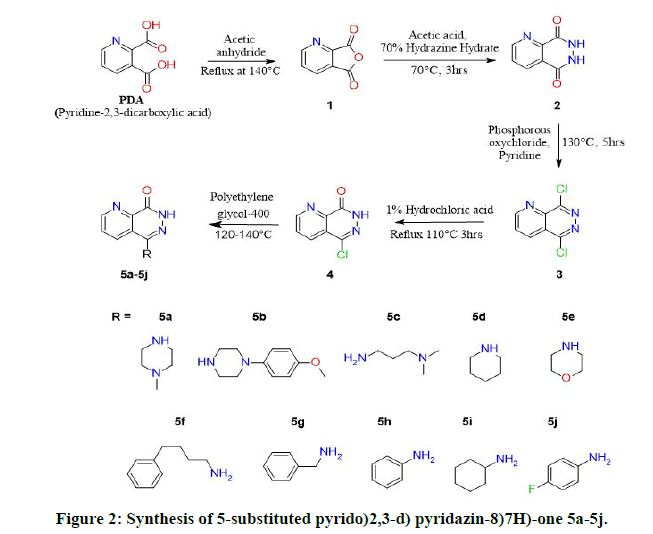
The synthesis of target molecules was carried out according to sequence of reaction that is outlined in Figure 2. The final compound 5-Chloropyrido(2,3-d)pyridazin-8(7H)-one (4) was obtained from dehydration of 2,3-Pyridine Dicarboxylic Acid (PDA) using acetic anhydride, the compound Furo(3,4-b)pyridine-5,7-dione (1) was then treated with hydrazine hydrate to obtain compound 6,7-dihydropyrido(2,3-d)pyridazine-5,8-dione (2) and then chlorinated with phosphorous oxychloride in the presence of pyridine to obtain 5,8-dichloropyrido(2,3-d)pyridazine (3), which was isolated by filtration and latter oxidized by heating with dilute hydrochloric acid (1%) to obtain 5-Chloropyrido(2,3-d)pyridazin-8(7H)-one (4) a key intermediate which was substituted with secondary amine derivatives in polyethyleneglycol-400 at 120 to 140°C on an oil bath [4]. The reaction process was monitored by using Thin Layer Chromatography(TLC) and each final compounds were purified by column chromatography with different concentration gradient ofethyl acetate and hexane [5].
The structures were elucidated by spectral studies including Infrared (IR), proton Nuclear Magnetic Resonance (1H-NMR) and LC-MS data confirmed the mass of synthesized compounds [6]. The IR spectra of all the synthesized 5-Chloropyrido(2,3-d) pyridazin-8(7H)-one derivatives were recorded in wave number (cm-1) [7]. The IR spectral strong peak of C-Cl was observed in 660-890 for compounds 3 and 4 which was absent for compounds 5a-5j due to substitution of amine group. The characteristic absorption band of aromatic ketone was observed at the range of 1,600 to 1,780 cm-1.The medium intense peaks of N-H stretching at the range of 3070-3500 was observed in final compounds 5a-5j,aromatic C-N stretching in the range of 1540-1680 cm-1. The 1H NMR spectra of 5a-5j displayed a singlet peak at 13.0-11.70 ppm attributed to NH-pyridazines. The 13C-NMR spectrum of compounds 5a to 5j represented by signal at δ 158ppm of owing to C=O of pyridazine ring. Mass spectrum of compound 4 showed a peak at m/z 182.0 [8].
Molecular docking
Virtual screening of all the compounds by PyRx predicted a significant binding affinity towards both voltages gated sodium channel receptor (PDB 6AGF) and NMDAR (PDB 5TP9). Phenobarbitone was used as the reference ligand for the comparison of binding affinity, with the active site binding energy being -6.7 kcal/mol and -6.1 kcal/mol (Table 1) [9]. Thus, the MES (Maximal Electro Shock) method for primary screening, and PTZ model of seizures as secondary screening. Compounds 5b, 5i and j exhibited a good binding affinity towards the receptor compare to Phenobarbitone (Figure 3) [10].
| Compound code | Binding energy (kcal/mol) | ||
|---|---|---|---|
| 5TP9 | 6AGF | ||
| 5a | -6.6 | -7.1 | -6.9 |
| 5b | -7.2 | -7.7 | -9.4 |
| 5c | -6.0 | -6.0 | -6.4 |
| 5d | -6.5 | -7.4 | -7.4 |
| 5e | -6.6 | -7.0 | -6.6 |
| 5f | -6.0 | -6.2 | -8.5 |
| 5g | -6.7 | -6.8 | -8.1 |
| 5h | -6.7 | -7.4 | -7.6 |
| 5i | -7.3 | -7.6 | -7.8 |
| 5j | -7.3 | -7.7 | -7.8 |
| Co-crystal ligand | -5.3 | -7.1 | -6.4 |
| Phenobarbitone | -6.0 | -6.1 | -6.7 |
Table 1: Binding energy of synthesized compounds with 5cOF, 5TP9 and 6AGF.
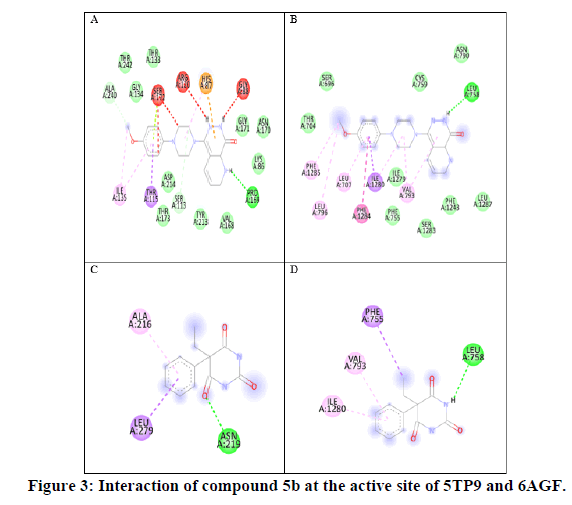
Figure 3 is 2D interaction of compound 5b (A) with NMDAR (5TP9) (B) with voltage gated sodium channel receptor (6AGF). Phenobarbitone (C) with NMDAR (5TP9) and interaction (D) with voltage gated sodium channel receptor (6AGF): hydrogen bonds are indicated by green dotted lines, hydrophobic interaction is represented by purple and red dotted lines [11].
Figure 1 show binding affinity of each synthesized molecules with respective receptors, compound 5b with substituted 4-methoxyphenyl group showed higher number of hydrophobic interactions bonds with the active site of sodiumchannel: 2 of them formed with isoleucine (Ile1280) between the piperidine ring and the phenyl ring. A significanthydrogen binding was found with leucine (Leu758) having a higher binding energy of -9.4 kcal/mol compare to the cocrystal ligand -6.4 kcal/mol and phenobarbitone -6.7 kcal/mol [12].
Pharmacological activity
Anticonvulsant activity (MES method): Compounds 5-(4-methylpiperazin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one (5a) and 5-(4-(4-methoxyphenyl)piperazin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one (5b) exhibited excellent activity with an 100% inhibition of HLTE (Hind Limb Tonic Extensor) phase along with compounds 5d and 5g also exhibiting 98.9% inhibition of convulsion produced by MES (Maximal Electro Shock) method. Whereas compounds 5c, 5e, 5f, 5i exhibited good anticonvulsant activity with percentage protection of 73.0, 74.2 78.7, and 93.3% respectively, in comparison to phenytoin which also exhibited 100% inhibition at the concentration of 25 mg/kg. The results are tabulated in the Table 2 [13].
| S.no | Compound code | Flexion (sec) | Extensor (sec) | Clonus (sec) | Stupor (sec) | % Inhibition |
|---|---|---|---|---|---|---|
| 1 | 5a | 6.1 | 0.0 | 1.8 | 69.3 | 100.0 |
| 2 | 5b | 7.3 | 0.0 | 7.0 | 57.2 | 100.0 |
| 3 | 5c | 5.3 | 4.0 | 5.0 | 113.7 | 73.0 |
| 4 | 5d | 2.2 | 0.2 | 4.5 | 79.2 | 98.9 |
| 5 | 5e | 3.5 | 3.8 | 6.7 | 123.5 | 74.2 |
| 6 | 5f | 9.7 | 3.2 | 3.7 | 111.7 | 78.7 |
| 7 | 5g | 2.2 | 0.2 | 0.0 | 109.7 | 98.9 |
| 8 | 5i | 6.0 | 1.0 | 9.3 | 95.5 | 93.3 |
| 9 | Control | 5.5 | 14.8 | 13.5 | 188.8 | 0.0 |
| 10 | Phenytoin | 2.8 | 0.0 | 33.2 | 35.2 | 100.0 |
Note: Compounds 5a and 5b exhibiting highest activity similar to the standard drug phenytoin
Table 2: Results of in-vivo anticonvulsant activity induced by MES method.
Anticonvulsant activity (PTZ model)
Pentylenetetrazol (PTZ) model used for the evaluation of petit mal type of epilepsy where the time period taken after the injection of PTZ to first two myoclonic jerks defined as seizure latency (Figures 4 and 5). The results of test compounds (25 mg/kg) were compared with that of diazepam (4 mg/kg) as a standard. Compound 5-(benzylamino)pyrido(2,3-d)pyridazin-8(7H)-on 5g exhibited an excellent activity by inhibiting 72.2% of convulsions produced, whereas compounds 5a, 5b, 5c, 5e and 5f showed a good activity with 57.4, 47.7, 46.6, 53.4, and 43.2% inhibition respectively [14]. The compounds 5d and 5i exhibited least activity. The mean duration of different phase convulsion induced by PTZ has been mentioned Table 3.
| S. no | Compound code | Onset of jerks (sec) | Onset of clonus (sec) | Duration of clonus (sec) | Onset of extensor (sec) | % Inhibition |
|---|---|---|---|---|---|---|
| 1 | 5a | 2.9 | 7.0 | 12.5 | 6.8 | 57.4 |
| 2 | 5b | 3.1 | 5.9 | 15.3 | 2.2 | 47.7 |
| 3 | 5c | 4.2 | 7.4 | 15.7 | 5.7 | 46.6 |
| 4 | 5d | 10.3 | 12.5 | 29.3 | 6.9 | 0.0 |
| 5 | 5e | 5.6 | 12.0 | 13.7 | 12.4 | 53.4 |
| 6 | 5f | 4.3 | 9.1 | 16.7 | 50.3 | 43.2 |
| 7 | 5g | 5.6 | 16.3 | 8.2 | 3.3 | 72.2 |
| 8 | 5i | 6.7 | 11.1 | 29.2 | 9.9 | 0.6 |
| 9 | control | 4.9 | 9.1 | 29.3 | 23.5 | 0.0 |
| 10 | Diazepam | 0.0 | 0.0 | 0.0 | 0.0 | 100.0 |
Note: Highest inhibition of convulsion was seen in compound 5g.
Table 3: Results of in vivo anticonvulsant activity induced by PTZ method.
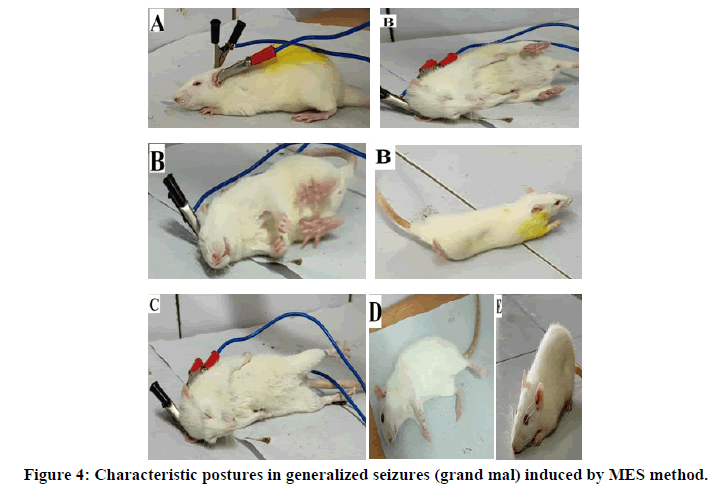
•Ear clip electrodes applied to induce the epilepsy.
•Tonic dorsal hyperextension of the neck and trunk, Tonic flexion (forward stretching) of forelimbs andstretching of the tail.
•Tonic extension of the hindlimbs
•Clonic convulsion (clonic movement of the trunk and four limbs).
•Stupor.
PTZ induced four behavioural phenomena.
•Freezing of body movement.
•Myoclonic twitches.
•Clonic seziures.(straub tail).
•Tonic clonic seziures.
Anti-inflammatory activity by denaturation Bovine Serum Albumin (BSA) assay
Anti-inflammatory activity of the synthesized compounds was determined at four different concentrations of 50 μg/ml, 100 μg/ml, 200 μg/ml and 500 μg/ml, which were compared against negative control (BSA solution only) and Indomethacin containing samples with similar concentration [15-19]. Compounds 5-(4-(4-methoxyphenyl)piperazin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one (5b) and 5-{(3-(dimethylamino)propyl)amino}pyrido(2,3-d)pyridazin-8(7H)-one (5c) showed an excellent activity by preventing 88.77% and 89.33% of denaturation of BSA protein induced by heat [20]. Compounds 5a, 5e, 5f, 5i, and 5j showed good activity by inhibiting 71.32, 81.40, 80.74, 77.27 and 77.79% of denaturation respectively. The compounds 5d, 5g, and 5h exhibited poor activity (Table 4).
| S. no | Compound code | Concentration | |||
|---|---|---|---|---|---|
| 50 μg | 100 μg | 200 μg | 500 μg | ||
| 1 | 5a | 19.12 | 32.89 | 60.8 | 71.32 |
| 2 | 5b | 10.64 | 50.0 | 85.7 | 88.77 |
| 3 | 5c | 17.33 | 69.33 | 80.0 | 89.33 |
| 4 | 5d | 2.64 | 5.66 | 8.3 | 26.42 |
| 5 | 5e | 9.3 | 30.23 | 51.16 | 81.4 |
| 6 | 5f | 16.05 | 42.86 | 53.61 | 80.74 |
| 7 | 5g | 7.04 | 11.27 | 25.35 | 38.03 |
| 8 | 5h | 5.93 | 26.58 | 44.74 | 58.89 |
| 9 | 5i | 9.09 | 60.61 | 81.06 | 77.27 |
| 10 | 5j | 27.41 | 72.59 | 65.15 | 77.78 |
| 11 | Indomethacin | 13.0 | 42.86 | 81.7 | 96.79 |
| Compound 5b and 5c showed a promising activity with highest percentage of protection of protein against denaturation | |||||
Table 4: Results of in vitro anti-inflammatory activity by BSA denaturation method.
Experimental section
All the chemicals were procured from Merck, Sigma Aldrich and Finar laboratories and were used without any additional purification. Melting points of all the compounds were determined by using open tube capillary in a digital melting point apparatus and were uncorrected. The reaction progress was monitored using TLC using Merck 0.25 mm silica gel plate time to time using mobile phase of ethyl acetate: hexane, and dichloromethane: methanol. Visualization of the developed TLC plates was accomplished by UV light 256 nm and 360 nm Synthesized compounds were purified using column chromatography.
The compounds were characterized by IR (ATR) and spectra were recorded by using potassium bromide (KBr) pellet technique on Shimadzu spectrometer. The 1H-NMR, spectra (chemical shift in ppm) were recorded in Dimethyl sulfoxide using Tetramethyl Silane (TMS) as an internal standard and 13C-NMR spectra using Dimethyl sulfoxide on Bruker Avance Ⅱ 300 NMR spectrometer and Bruker Avance Ⅱ 400 NMR spectrometer respectively.
Synthesis of Furo(3,4-b) pyridine-5,7-dione and 6,7-Dihydropyrido(2,3-d) pyridazine-5,8-dione(1-2)
0.598 mol of 2,3-Pyridine Dicarboxylic Acid (PDA-1) was suspended in 250 ml of acetic acid in a two neck round bottom flask at room temperature and refluxed at 110°C with constant stirring for 3-4 hours and cooled to room temperature. 500 ml of Dichloromethane was added at 0°C-5°C to extract the product by precipitation and followed by drying at reduced pressure. 0.469 mol Furo(3,4-b) pyridine-5,7-dione (1).
Furo(3,4-b) pyridine-5,7-dione (1) collected was reacted with 32 g hydrazine hydrate added in a dropwise manner under open condition and refluxed at 95°C for 3 hours to obtain white colored 6,7-dihydropyrido(2,3-d) pyridazine-5,8-dione (2)a white amorphous solid with yield 85%; melting point (mp) 160.0-190.0°C. 1H NMR (DMSO, 400 MHz) δ 13.13(2H, s, NH), 9.20 (1H, m, J=30.5 hz, H-2), 8.61 (1H, q, J=8.0 Hz, H-4), 8.40 (1H, q, J=9.6, H-3). 13C NMR (DMSO, 300MHz) δ 158.82 (C-8), 154.0 (C-5), 149.3 (C-2), 143.1 (C-9), 135.0 (C-4), 125.9 (C-3), 124.9 (C-10). CHN analysiscalcd for C7H5N3O2 C (51.54%) H (3.09%) N (25.76%) found C (51.68%) H (3.01%) N (25.79%).
Synthesis of 5,8-Dichloropyrido(2,3-d) pyridazine(3) and 5-Chloropyrido(2,3-d) pyridazin-8(7H)-one(4)
0.399 mol of 7-dihydropyrido(2,3-d) pyridazine-5,8-dione and 1.95 mol of phosphorous oxychloride react in presence of pyridine (0.8 mol) to give the product of 5,8-dichloropyrido(2,3-d) pyridazine (3) and it was refluxed with dilute hydrochloric acid to precipitate 5-Chloropyrido(2,3-d) pyridazin-5(6H)-one (4). A grey colored amorphous solid obtained from recrystallization with the acetic acid. Yield 85%; melting point (mp) 271.0-274.9˚C, FTIR (KBr) νmax; 3155.2(N-H str), 1233.7(Ar C-N str), 1705(C=O str), 1587.8(Ar C-H str), 814.4 cm-1(-Cl str). 1H NMR (DMSO, 400 MHz) δ 13.08 (1H, s, NH), 9.20 (1H, m, J=30.5 hz, H-2), 8.61 (1H, q, J=8.0 Hz, H-4), 8.40 (1H, q, J=9.6, H-3). 13C NMR (DMSO, 300 MHz) δ 154.10 (C-8), 158.9 (C-5), 148.3 (C-2), 142.86 (C-9), 135.1 (C-4), 126.2 (C-3), 125.0 (C-10). CHN analysis calcd for C7H5clN3O C (46.30%) H (2.22%) N (23.14%) found C (46.36%) H (2.31%) N (23.14%).
General procedure to synthesis of 5-subsituted pyrido(2,3-d) pyridazine--8(7H)-one
0.02 mol 5-Chloropyrido(2,3-d) pyridazin-8(7H)-one in 15 ml of polyethylene glycol 400 was treated with 0.13 mol amine derivatives. The reaction mixture was heated to 140˚C for appropriate time in an oil bath. The reaction mixture mass poured into ice-water mixture and extracted with dichloromethane. The organic phase was separated, washed with water, dried with sodium sulfate, and evaporated to dryness and the crude product thus obtained is purified by column chromatography (Hexane: Ethylacetate).
5-(4-methylpiperazin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one(5a)
Brown colored crystalline solid obtained from methanol to yield 24%; mp 220.0-230.3˚C, λmax=300 nm; FTIR(KBr) νmax; 3160.8(N-H str), 1358.6(C-N str) 1660.5(C=O str), 1597.2(Ar C-H). 1H NMR (DMSO, 300 MHz) δ 12.28 (1H, s, NH), 9.12 (1H, d, J=4.2 Hz, H2), 8.60 (1H, d, J=8.1 Hz, H4), 7.84 (1H, q, J=12, H3), 3.51 (5h, s, piperazine H3, H5), 2.72 (5h, s, piperazine H2, H6), 2.38 (3H, s, CH3). 13C NMR (DMSO, 400 MHz) δ 158.9(C-8), 154.1(C-5), 148.2(C-2), 142.8(C-9), 135.1(C-4), 126.2(C-3), 125.0(C-10), 69.79(C-3, C-5 piperazine), 53.8(C-2 piperazine), 48.4(C-6 piperazine), 40.14(CH3). LCMS m/z calcd. for C12H15N5O 246.13(M+1)+ found: 246.2(M+1)+, CHN analysis calcd for C12H15N5O C(58.76%) H(6.16%) N(28.55%), found C(58.76%) H(6.16%) N(28.55%).
5-(4-(4-methoxyphenyl)piperazin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one(5b)
Brown colored crystalline solid obtained from ethanol to yield 43%; mp 240- 272˚C, λmax=243 nm; FTIR (KBr) νmax; 3160.8(-NH str), 1660.5(C=O), 1246.8(C-O-C str), 1513.3(Ar C-H str), 1448(-CH bending), 1265(C-N str). 1H NMR(DMSO, 400 MHz) δ 12.27 (1H, s, NH), 9.13 (1H, d, J=4.4 Hz, H2), 8.60(1H, m, J=10 Hz, H4), 7.85(1H, m, J=10.4, H3), 6.97(2H, d, J=7.2 Hz phenyl H3, H5), 6.84(2H, d, phenyl H2, H6), 3.57(5h, s, piperazine H3, H5), 3.19(5h, s, piperazine H2, H6), 3.69(3H, d, J=2 Hz CH3). 13C NMR (DMSO, 300 MHz) δ 158.8(C-8), 154.0(C-5), 153.0(C-1 phenyl) 148.4(C-2), 145.4(C-9), 135.1(C-4), 126.1(C-3), 125.0(C-10), 117.5(C-2, C-6 phenyl), 114.2(C-3, C-5 phenyl), 49.5(C-2, C-6 piperazine), 49.31(C-2, C-6 piperazine), 55.18(CH3). LCMS m/z calcd. for C18H19N5O2 Calcd: (m/z): 338.16(M+1)+ Found: 338.3(M+1)+: Anal. Calcd. for C18H19N5O2 C(64.08%), H(5.68%), N(20.76%), found C(64.08%), H(5.68%), N(20.76%).
5-{(3-(dimethylamino)propyl)amino}pyrido(2,3-d)pyridazin-8(7H)-one(5c)
Yellowish green amorphous solid obtained methanol to yield 29%; mp 110- 125˚C, λmax=243 nm; FTIR(KBr) νmax; 3166.4(secondary amide -NH str), 3401.2(secondary amine -NH str), 1658.7(C=O str), 1584.1(C-O-CH str), 1597.2(Ar C-H str), 1440.6(-CH bending), 1099.6(C-N str), 2702.3(C-H str). 1H NMR(DMSO, 400 MHz) δ 11.75(1H, s, NH),9.07(1H, q, J=6.4 Hz, H2), 8.56(1H, q, J=10 Hz, H4), 7.87(1H, q, J=12.4, H3), 6.96(1H, t, J=11.6 Hz, NH- DMAPA),3.496(5h, m, DMAPA H2, H3, H6, H7), 2.82(2H, d, J=5.2 Hz CH3), 2.66(5h, d, J=1.6 Hz), 2.48(2H, s, DMAPA H4, H5).13C NMR(DMSO, 300 MHz) δ 157.7(C=O), 154.1(C-2), 14.4(C-9), 145.5(C-5), 135.0(C-4), 126.7(C-3), 123.8(C-10),72.35(C-3 DMAPA), 60.20(C-1 DMAPA), 24.08(C-2 DMAPA), 42.82(-CH3), 55.35(-CH3). LCMS m/z calcd. forC12H17N5O Calcd: (m/z): 247.14(M)+ Found: 247.93(M)+. Anal. Calcd. for C12H17N5O C(58.28%) H(6.93%) N(28.32%)found C(58.28%) H(6.94%) N(28.31%).
5-(piperidin-1-yl)pyrido(2,3-d)pyridazin-8(7H)-one(5d)
White amorphous solid from hexane to yield 47%; mp 210- 225˚C; λmax 263 nm; FTIR(KBr); νmax; 3019.1(-NH str), 1658.7(C=O str), 1571.1(Ar C-H str), 1440.6(C-H bending), 2931.6(C-H str), 1960.6(Ar C-H bending). 1H NMR(DMSO, 300 MHz) δ 12.17 (1H, s, NH), 9.11 (1H, d, J=3 Hz, H2), 8.58 (1H, t, J=7.8 Hz, H4), 7.83 (1H, q, J=12.6, H3), 3.38 (5h, q, J=20.4 Hz, piperidine H1, H2, H9, H10), 2.72 (2H, m, piperidine H5, H6), 1.68(5h, d, J=25 Hz piperidine H3, H4, H7, H8). 13C NMR(DMSO, 300 MHz) δ 158.8 (C=O), 149.3(C-2), 143.1(C-9), 154.0(C-5), 135.0(C-4), 125.9(C-3), 124.9(C-10), 50.4(C-2, C-6 piperidine), 25.33(C-3, C-5 piperidine), 24.17(C-4 piperidine). LCMS m/z calcd. for C12H14N4O Calcd: (m/z): 231.12(M+1)+ Found: 231.2(M+1)+ Anal. Calcd. for C12H14N4O C(62.59%) H(6.13%) N(24.33%) found C(62.59%) H(6.17%) N(24.34%).
5-(morpholin-4-yl)pyrido(2,3-d)pyridazin-8(7H)-one(5e)
White amorphous obtained solid from ethyl acetate to yield 64%; mp 210- 240˚C; λmax = 264 nm; FTIR(KBr) νmax; 3157.1(-NH str), 1690.3(C=O str), 1595.3(Ar C-H str), 1487-1351(-CH bending), 1120.1(C-O-C str), 2935.3(C-H str). 1H NMR(DMSO, 300 MHz) δ 12.27(1H, s, NH), 9.12(1H, d, J=6.3 Hz, H2), 8.60(1H, m, J=9.6 Hz, H4), 7.87(1H, m, J=16.5, H3), 3.80(5h, t, J=9 Hz, morpholine H1, H2, H9, H10), 3.50(5h, s, morpholine H5, H6, H7, H8). 13C NMR(DMSO, 400 MHz) δ 158.9(C=O), 148.3(C-2), 142.8(C-9), 154.1(C-5), 135.1(C-4), 126.2(C-3), 125.0(C-10), 66.04(C-2, C-6 morpholine), 49.83(C-3, C-5 morpholine). LCMS m/z calcd. for C11H12N4O2 Calcd:(m/z): 232.09(M)+ Found: 232.89(M)+ Anal. Calcd. for C11H12N4O2 C(56.89%) H(5.21%) N(24.12%) found C(56.90%) H(5.21%) N(24.17%).
5-( (4-phenylbutyl)amino)pyrido(2,3-d)pyridazin-8(7H)-one(5f)
White amorphous solid obtained from ethyl acetate to yield 24%; mp 170-180˚C; λmax=272 nm; FTIR(KBr) νmax; 3404.9, 3157.1(-NH str), 1662.4(C=O), 2927.8, 28994.3(C-H str), 1582.3(Ar-CH str), 1530.1(-C=C str), 1459-1477(-CH bending). 1H NMR(DMSO, 300 MHz) δ 11.70(1H, s, NH), 9.06(1H, q, J=6.3 Hz, H2), 8.56(1H, m, J=9.9 Hz, H4), 7.87(1H, q, J=12.6, H3), 7.28(5H, m, J=39.9 Hz phenyl), 6.73(1H, t, J=11.4 Hz, NH phenylbutylamine), 2.50(3H, t, J=3.3 phenylbutylamine H2, H3, H8), 1.64(5h, t, J=6.9 phenylbutylamine H4, H5, H6, H7). 13C NMR (DMSO, 400 MHz) δ 157.6(C=O), 145.5(C-2), 142.1(C-9), 153.9(C-5), 135.0(C-4), 125.5(C-3), 123.7(C-10), 128.2(C-2, C-4, C-5 phenyl), 126.5(C-3 phenyl), 140.7(C-1 phenyl), 34.91(C-2, C-3 phenylbutylamine), 28.64(C-3, C-4 phenylbutylamine). LCMS m/z calcd. for C17H18N4O Calcd: (m/z): 295.15(M+1)+ Found 295.2(M+1)+. Anal. Calcd. for C11H12N4O2 C(69.37%) H(6.16%) N(19.03%) found C(69.35%) H(6.16%) N(19.01%).
5-(benzylamino)pyrido(2,3-d)pyridazin-8(7H)-one(5g)
White crystalline solid obtained from ethanol to to yield 25%; mp 170-185˚C; λmax=342 nm; FTIR(KBr) νmax; 3378.8(-NH str), 1662.4(-C=O str) 2922.2, 2959.5(C-H str), 1522.6(Ar-CH str), 1530(-C=C str), 1459-1477(-CH bending). 1H NMR(DMSO, 300 MHz) δ 11.71(1H, s, NH), 9.10(1H, d, J=1.8 Hz, H2), 8.58(1H, q, J=8.1 Hz, H4), 7.91(1H, m, J=12.6, H3), 7.34(6H, m, J=40.5 Hz phenyl), 4.52(2H, d, J=3.9 Hz, NH benyl amine). 13C NMR(DMSO, 400 MHz) δ 157.6(C=O), 145.5(C-2), 142.1(C-9), 153.9(C-5), 135.0(C-4), 125.5(C-3), 123.7(C-10), 128.2(C-2, C-4, C-5 phenyl), 126.5(C-3 phenyl), 140.7(C-1 phenyl), 34.91(C-2, C-3 phenylbutylamine), 28.64(C-3, C-4 phenylbutylamine). LCMS m/z calcd. for C15h12N4O Calcd: (m/z): 253.10(M+1)+ Found 253.1(M+1)+. Anal. Calcd. for C15h12N4O C(66.65%) H(4.79%) N(22.21%) found C(66.66%) H(4.76%) N(22.23%).
5-(cyclohexylamino)pyrido(2,3-d)pyridazin-8(7H)-one(5h)
Brown amorphrous solid obtained from methanol to yield 22%; mp=180-183˚C; λmax=272 nm; FTIR(KBr) νmax: 3166.4(-NH str), 1697.8(C=O str), 1528.2(Ar-CH str), 1446.2(C-H bending), 1310.2(C-H str), 1010.1(C-N str). 1H NMR(DMSO, 400 MHz) δ 13.08(1H, s, NH), 9.20(1H, q, J=6.0 Hz, H2), 8.62(1H, q, J=8 Hz, H4), 7.95(1H, q, J=12.4, H3), 3.79(5h, m, cyclohexyl), 2.31(6H, m,cyclohexyl). 13C NMR(DMSO, 400 MHz) δ 159.9(C=O), 144.5(C-2), 143.4(C-9), 155.9(C-5), 135.1(C-4), 125.2(C-3), 123.9(C-10), cyclohexylamine- 49.02(C-1), 46.02(C-2), 34.20(C-6), 32.32(C-3), 29.05(C-5), 25.43(C-4). LCMS m/z calcd. for C13H16N4O Calcd: (m/z): 245.13(M+1)+ Found 245.1(M+1)+. Anal. Calcd. for C13H16N4O C(63.91%) H(6.60%) N(22.93%) found C(63.89%) H(6.65%) N(22.96%).
5-anilinopyrido(2,3-d)pyridazin-8(7H)-one(5i)
Brown amorphrous solid obtained from methanol to yield 30%; mp=240-265˚C; λmax=291 nm; TIR(KBr) νmax: 3356.5)-NH str), 1669.8(C=O str), 1591.3(Ar C-H str), 1427(C-H bending), 1310.2(C-N str). 1H NMR(DMSO, 300 MHz) δ 13.09(1H, s, NH), 9.21(1H, m, J=13.8 Hz, H2), 8.93(1H, s, H4), 8.63(1H, q, J=9.0, H3), 7.96(2H, t, J=13.5 Hz phenyl), 7.87(1H, d, J=7.8 Hz phenyl), 7.33(1H, t, J=16.2 Hz phenyl), 6.96(2H, s, phenyl), 3.33(1H, s, NH aniline). 13C NMR(DMSO, 400 MHz) δ 159.6(C=O), 143.4(C-2), 142.6(C-9), 155.8(C-5), 135.2(C-4), 125.1(C-3), 124.2(C-10), Phenyl- 138.9(C-1), 128.5(C-2), 127.5(C-6), 121.28(C-3), 126.9(C-5), 118.5(C-4). LCMS m/z calcd. for C13H10N4O Calcd:(m/z): 238.08(M)+ Found 238.95(M)+. Anal. Calcd. for C13H10N4O C(65.54%) H(4.23%) N(23.52%) found C(65.53%) H(4.28%) N(23.54%).
5-(4-fluoroanilino)pyrido(2,3-d)pyridazin-8(7H)-one(5j)
Purple amorphous solid obtained from methanol to yield 40%; mp=245-270˚C; λmax=258 nm; FTIR(KBr) νmax; 3375.1(-NH str), 1686.6(C=O str), 1586.0(Ar-CH str), 1444.3(CH bending), 1310.2(C-N str), 1010.1(C-F str). 1H NMR(DMSO, 300 MHz) δ 13.09(1H, s, NH), 9.21(1H, m, J=13.8 Hz, H2), 8.93(1H, s, H4), 8.63(1H, q, J=9.0, H3), 7.96(2H, t, J=13.5 Hz phenyl), 7.87(1H, d, J=7.8 Hz phenyl), 7.33(1H, t, J=16.2 Hz phenyl), 6.96(2H, s, phenyl), 3.33(1H, s, NH aniline). 13C NMR(DMSO, 100 MHz) δ 159.6(C=O), 143.4(C-2), 142.4(C-9), 155.9(C-5), 135.2(C-4), 125.2(C-3), 124.2(C-10), Phenyl-138.9(C-1), 127.6(C-2), 127.0(C-6), 120.39(C-3), 124.2(C-5), 115.1(C-4). LCMS m/z calcd. for C13H9FN4O Calcd: (m/z): 257.08(M+1)+ Found 257.0(M+1)+. Anal. Calcd for C13H9FN4O C(60.94%) H(3.54%) F(7.41%) N(21.87%) found C(60.93%) H(3.54%) N(21.82%).
Pharmacological activity
Anticonvulsant activity: The protection against the convulsions by synthesized compounds were studied in-vivo against MES-induced seizures and pentylenetetrazol induced seizures. Swiss albino rats weighing 150-200 g (n=6) of female sex were used in the experimentation. The animals were housed under a well-maintained and controlled conditions of light/dark schedule (12 hr) and temperature 25 ± 1˚C in a polypropylene cage with a dust free rice husk as a bedding. Animals had free access to food and water ad libitum. Before subjecting to the study, the animals were given a week of time to acclimatized with the laboratory condition. All the procedures for the pharmacological activities were priorly reviewed and approved by IAEC (Institutional Animal Ethical Committee) with reference no. AACP/IAEC/SEP2021/08(Approved by CPCSEA Regd No. 83/ReBi/SS/99/CPCSEA).
Anticonvulsant activity (MES method)
The anticonvulsant activity of the test drugs (synthesized pyridazine compounds) against grand mal type of epilepsy was determined by using MES (Maximal Electro shock) method (Model: Techno Electro Convulsometer). Test compounds and phenytoin was injected intraperitonially (i.p) at dose level 25 mg/kg body weight to each animal of different groups. A freshly prepared solution of solution of 5% gum acacia was used as a vehicle. Control animals were treated with vehicle only. The convulsions were induced 30min after drug treatment by application of ear clip electrode passing an alternating current of 50 mA for 0.2 sec. The abolition of the Hind Limb Tonic Extensor (HTLE) spasm was recorded as a measure of anticonvulsant activity. The results are tabulated in Table 2.
Anticonvulsant activity (PTZ model)
Pentylenetetrazol (PTZ) 80 mg/kg of body weight was administered subcutaneously into the scruff of the neck the animals. Diazepam was used as positive control at dose of 4 mg/kg body weight to each animal of different group of test drugs containing 6 animals each. test drug at a dosage of 25 mg/kg body weight was administered i.p, suspended in 5% gum acacia as pentylenetetrazol and diazepam respectively. Control animals were treated with vehicle only. PTZ was administered after 0.5 hr of administration of Diazepam i.p, in the standard group and test compounds in the test group of animals. The onset and severity of the myoclonic jerks and generalized seizure were recorded and tabulated in Table 2. The death of animals during the procedure was also recorded.
Anti-inflammatory activity by denaturation bovine serum albumin assay
The anti-inflammatory activity of the synthesized target compounds was determined by denaturation of the bovine serum albumin technique as per Mizushima and Kobayashi with slight modification. The sample to be analyzed were taken in 5ml total volume which contain 2% aqueous solution of bovine serum albumin and the pH of reaction mixture was adjusted to 7.4 using phosphate buffer saline. The test samples were further incubated for 30 mins at 370 C, and then heated to 510 C for 20 mins and cooled to room temperature and the solution was measured at 660 nm using UV-visible spectrometer. Percentage of inhibition of denaturation was calculated with reference to standard solutions of Ibuprofen and control samples with BSA and were tabulated in Table 3. The percentage inhibition of denaturation was calculated by using following formula,
%Inhibition=(Absorbance of control-Absorbance of test)/(Absorbance of control)*100
Molecular docking
ChemDraw ultra 12.0 software was used to prepare the ligand chemical structure, and were saved in mol format. The structures were later optimized by Chem3D Pro by Molecular mechanical MM2 algorithm, the 3D structures of the proteins were downloaded from RCSB Protein Data Bank (RCSB PDB). The binding interaction of all the compouds
determined with the protein targets NMDAR is an ionotropic glutamate receptor (PDB ID: 5TP9), gated by the endogenous coagonists glutamate and glycine, Gamma-aminobutyric acid receptor complexed with co-crystalized Benzamidine (PDB ID: 5cOF), and Voltage-gated sodium channel (PDB ID: 6AGF) co-crystallized with (2~{R})-1-(2-azanylethoxy)oxidanyl)phosphoryl) oxy-3-hexadecanoyloxy-propan-2-yl))~{Z})-octadec-9-enoate).
PyRx(AutoDock Vina 4.0 and AutoDock Vina) was used to predict binding energy of compounds by Empirical Fee Energy Scoring function and Lamarckian Genetic Algorithm. Macromolecules were prepared for docking by removing water molecules and with addition of the polar groups. The cocrystal molecule was isolated and hydrogen molecule were addedd to stabilise the molecule. active site was identified by cocrystal ligand and the grid box was placed to fit the binding site. The protein ligand interaction of compounds with respective ligand was visualized by Discovery Studio software.
CONCLUSION
The successful synthesis of novel 5-substituted amino pyridazines and their screening by in silico and in vivo anticonvulsant activities of compound 5a and 5b exhibited 100% in comparison with Phenytoin and compound 5g exhibiting a considerable potent anticonvulsant activity as well as the considerable anti-inflammatory activity by compound 5c showed that these derivatives may be used as a lead compound for developing newer anticonvulsant drugs.
ACKNOWLEDGMENT
Authors are much obliged to convey their gratitude to Anthem Bioscience Pvt Ltd, for providing continuous support and necessary facilities in carrying out the synthetic work. Authors extend their gratitude to Principal, Al-Ameen college of Pharmacy and Department of Pharmaceutical chemistry for their support. A special thanks to Mr. Rakesh Panda Associate Professor, Al-Ameen college of Pharmacy for their support.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
References
- Jampilek J. Molecules. 2019;24:3839.
[Crossref] [Google Scholar] [PubMed]
- Arora P, et al. Int J Pharm Sci Res. 2012;3:2947-2954.
- Bansal R, et al. Med Chem Res. 2013;22:2539-2552.
- Khan A, et al. Molecules. 2020;25:2002.
[Crossref] [Google Scholar] [PubMed]
- Mishra R, et al. J Chil Chem Soc. 2011;56:856-859.
- Rathish IG, et al. Eur J Med Chem. 2009;44:2673-2678.
[Crossref] [Google Scholar] [PubMed]
- Kamble VT, et al. Arch Pharm Chem Life Sci. 2015;348:338-346.
[Crossref] [Google Scholar] [PubMed]
- Sabt A, et al. J Enzyme Inhib Med Chem. 2020;35:1616-1630.
[Crossref] [Google Scholar] [PubMed]
- Moghimi S, et al. Bioorg Chem. 2021;109:104670.
[Crossref] [Google Scholar] [PubMed]
- Castro M, et al. Eur J Med Chem. 1994;29:831-839.
- Abdel Gawad NM, et al. Med Chem Res. 2011;20:1280-1286.
- Asif M. Ref J Chem. 2018;8:280-300.
- Singh J, et al. Future Med Chem. 2017;9:95-127.
[Crossref] [Google Scholar] [PubMed]
- KM F, et al. Neurology. 2017;89:1-642.
[Crossref] [Google Scholar] [PubMed]
- Deckers CLP, et al. Epilepsy Res. 2003;53:1-17.
[Crossref] [Google Scholar] [PubMed]
- Wahab A. Pharmaceuticals (Basel). 2010;3:2090-2110.
[Crossref] [Google Scholar] [PubMed]
- Sharma B, et al. Med Chem Res. 2014;23:146-157.
- Asif M, et al. Iran J Pharm Sci. 2013;9:67-80.
- Zayed M, et al. Arzneimittelforschung. 2012;62:532-536.
[Crossref] [Google Scholar] [PubMed]
- Bian M, et al. J Enzyme Inhib Med Chem. 2013;28:792-800.
[Crossref] [Google Scholar] [PubMed]