Original Articles: 2025 Vol: 17 Issue: 1
Synthesis and Characterization of Adducts of Bis(o-isobutyldithiocarbonato) Copper (II) with Substituted Pyridines and their Biological Studies
Neerupama1, Rajinder Singh1*, Renu Sachar2
1 Department of Applied Sciences, Govt. College for Engineering and Technology, Jammu & Kashmir, India
2 Department of Chemistry, University of Jammu, Jammu & Kashmir, India
*Corresponding Author:
- Rajinder Singh Department of Applied Sciences, Govt. College for Engineering and Technology, Jammu & Kashmir, India
Received: 10-Aug-2023, Manuscript No. JOCPR-23-110116; Editor assigned: 12-Aug-2023, PreQC No. JOCPR-23-110116 (PQ); Reviewed: 26-Aug-2023, QC No. JOCPR-23-110116; Revised: 23-Jan-2025, Manuscript No. JOCPR-23-110116 (R); Published: 30-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).231.
Citation:Neerupama, et al. 2025. Synthesis and Characterization of Adducts of Bis(o-isobutyldithiocarbonato) Copper (II) with Substituted Pyridines and their Biological Studies. J. Chem Pharm. Res., 17:231.
Abstract
A series of adducts derived from Bis(O-isobutyldithiocarbonato) copper(II) and substituted pyridines has been successfully synthesized. This was achieved by reacting Bis(O-isobutyldithiocarbonato) copper(II) with various substituted pyridines in equimolar proportions, using acetone as the solvent. Through comprehensive analytical investigations, it has been determined that the resulting adducts exhibit a consistent 1:1 stoichiometry, with a general molecular formula of Cu(O-isobutyldithiocarbonato) 2L, where L represents 2 and 3-bromopyridine, 4- acetylpyridine, 3-hydroxypyridine, 2-methoxypyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, and 2- amino-5-methylpyridine. The adducts have been subjected to a thorough characterization process, involving elemental analysis, conductivity measurements, magnetic susceptibility measurements, as well as infrared and electronic spectral studies. These comprehensive analyses collectively indicate the adoption of a trigonal bipyramidal structural arrangement by the adducts. Furthermore, the adducts have been identified as possessing a paramagnetic nature. Additionally, an evaluation of the antifungal properties of selected adducts against the Fusarium oxysporium fungal strain has been undertaken. This research serves to provide valuable insights into the potential applications of these adducts in the field of antifungal agents.
Keywords
Bis(O-isobutyldithiocarbonato) copper(II), Substituted pyridines, Characterization process, Involving elemental analysis, Conductivity measurements, Magnetic susceptibility measurements
Introduction
Dithiocarbamates, xanthates, and dithiocarboxylates are important members of the 1,1-dithiolate family that have garnered significant research attention from chemists, physicists, and biologists. This is due to their diverse applications and intriguing biological, structural, magnetic, electrochemical, and thermal activities. Xanthates have found successful use as fungicides, pesticides, vulcanization accelerators, flotation agents, corrosion inhibitors, highpressure lubricants, and, more recently, in the therapy of HIV infection. Both xanthates and thioxanthates represent two promising novel classes of inhibitors for human cancer [1].
Metallic xanthates are well-established reagents in the flotation of minerals and transition metals such as copper, zinc, and nickel [2]. They are also employed in the separation and quantitative determination of a wide range of cations. Metallo-xanthate anions have shown promise as potential ligands for preparing heterobimetallic xanthates, which could serve as precursors for heterobimetallic sulfide materials [3]. Sodium and potassium ethyl xanthates have demonstrated antidotal effects in cases of acute mercury poisoning. Transition metal xanthate complexes have been investigated for their potential applications in nonlinear optics [4].
Materials and Methods
Experimental
The potassium salts of O-alkyldithiocarbonates were prepared by the standard published method
Preparation of potassium butyldithiocarbonate
The potassium salt of O-butyldithiocarbonate was prepared using the standard published method [5]. In a 500 ml round-bottomed flask equipped with a reflux condenser, 4.2 g (0.075 mol) of potassium hydroxide pellets were placed, and 19.271 g (23.472 ml, 0.26 mol) of n-butanol was added [6]. The reaction mixture was heated under reflux for 1 hour. After cooling, the liquid from the reaction mixture was decanted into another dry 500 ml flask. To this flask, 5.7 g (4.5 ml, 0.075 mol) of carbon disulfide was slowly added with continuous heating. After cooling the flask in ice, the contents were filtered through a sintered glass funnel using a pump and washed with three 25 ml portions of ether. The resulting product, potassium O-butyldithiocarbonate, was dried in a vacuum desiccator with anhydrous calcium chloride [7].
Preparation of complexes
All attempts aimed at isolating stable complexes of bis(O-alkyldithiocarbonato)copper(II) have resulted in the isolation of the corresponding bis(O-alkyldithiocarbonato)copper(I) compounds. However, instead of pursuing the parent compounds, a different approach was taken [8]. By mixing the reactants–cupric chloride dihydrate, potassium salts of O-alkyl dithiocarbonates, and nitrogen donor ligands in a 1:2:1 ratio in acetone, the formation of stable adducts of bis(O-alkyldithiocarbonato) copper(II) was observed. This observation was confirmed through various physico-chemical techniques and spectroscopic studies [9].
Preparation of the adducts
A solution of CuCl2·2H2O (0.42 g, 0.0026 mol) was prepared in acetone. To this solution, a solution of the potassium salt of O-isobutyldithiocarbonate (0.74 g, 0.0052 mol) and the respective substituted pyridine (0.0026 mol) was added. The substituted pyridine solution was prepared using 50 ml of acetone [10]. The resulting mixture was stirred for 30 minutes, during which green precipitates formed. These precipitates were promptly filtered and subsequently dried in a vacuum desiccator containing anhydrous calcium chloride [11]. Several different substituted pyridines were employed in the reaction: 2-bromopyridine (0.410 g), 3-bromopyridine (0.410 g), 4-acetylpyridine (0.314 g), 3- hydroxypyridine (0.247 g), 2-methoxypyridine (0.287 g), 2-amino-3-methylpyridine (0.2511 g), 2-amino-4- methylpyridine (0.2511 g), and 2-amino-5-methylpyridine (0.2511 g) (each equivalent to 0.0026 mol) [12]. The reaction mixture was allowed to stand undisturbed for a period of 20-24 hours. Subsequently, the green product obtained was washed using the same solvent employed in its preparation and then dried over calcium chloride at room temperature [13].
Methods
Elemental analysis for carbon, hydrogen, nitrogen, and sulfur was conducted using an elemental analyzer (Elementar Vario EL III, Carlo Erba 1108). Molar conductance measurements were performed on millimolar solutions in DMF (dimethylformamide) utilizing the century CC 601 conductivity bridge [14]. Infrared spectra of the complexes spanning the range of 4,000 to 200 cm^(-1) were recorded using KBr pellets on an Infrared spectrophotometer (Perkin Elmer FT-IR).
Electronic spectra of the adducts were recorded in DMF on a Systronics 119 UV-visible spectrophotometer. Magnetic moments were determined at room temperature using the VSM (Vibrating Sample Magnetometer) method with the Princeton Applied Research Model No. 155. The analytical data, molar conductance values, and magnetic moments of the isolated adducts are presented in the accompanying tables [15].
Resulits And Discussion
Preliminary investigations
The adducts formed between bis(O-isobutyldithiocarbonato)copper(II) and various nitrogen donor ligands exhibit a distinctive bright green microcrystalline solid appearance. Elemental analysis discloses that these isolated adducts adhere to a 1:1 stoichiometry with a general formula of (Cu(S2COC4H9)2(L)), where (L) represents the nitrogen donor ligands 2- and 3-bromopyridine, 4-acetylpyridine, 3-hydroxypyridine, 2-methoxypyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, and 2-amino-5-methylpyridine. While these adducts prove to be insoluble in commonly used organic solvents like acetone, 1,4-dioxane, nitrobenzene, ethanol, and methanol, they do exhibit solubility in specific organic solvents such as dimethyl sulfoxide and dimethylformamide. In contrast, the parent complexes, namely copper(II)dithiocarbonates, are notably unstable and tend to reduce to their corresponding copper(I)dithiocarbonates [16]. However, the synthesized adducts demonstrate a higher degree of stability when exposed to air. Various physico-chemical analyses indicate that, when compared to the parent complexes, specifically copper(II)xanthates which are prone to instability and quick reduction to their corresponding copper(I)xanthates, the synthesized adducts exhibit a relatively robust stability in atmospheric conditions. The analytical data concerning the adducts of bis(O-alkyldithiocarbonato)copper(II) with the assorted nitrogen donor ligands have been consolidated and presented in Table 1 [17].
| S. no | Name of the adduct | Mol.Wt. | % Yield | Colour | %age (Found) | %age (Calculated) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| C | H | N | S | C | H | N | S | |||||
| 1 | Bis(O-isobutyldithiocarbonato) (2-bromopyridine)copper(II) |
519.4 | 76 | Green | 33.9 | 4.01 | 2.16 | 24.11 | 34.65 | 4.23 | 2.69 | 24.64 |
| 2 | Bis(O-isobutyldithiocarbonato) (3-bromopyridine)copper(II) |
519.4 | 84 | Green | 34.11 | 3.99 | 2.2 | 24.15 | 34.65 | 4.23 | 2.69 | 24.64 |
| 3 | Bis(O-isobutyldithiocarbonato) (4-acetylpyridine)copper(II) |
482.5 | 81 | Green | 41.8 | 5.12 | 2.15 | 26.01 | 42.28 | 5.39 | 2.9 | 26.52 |
| 4 | Bis(O-isobutyldithiocarbonato) (3-hydroxypyridine)copper(II) |
457.5 | 79 | Green | 38.85 | 4.97 | 2.95 | 27.21 | 39.34 | 5.24 | 3.06 | 27.98 |
| 5 | Bis(O-isobutyldithiocarbonato) (2-methoxypyridine)copper(II) |
470.5 | 83 | Green | 39.94 | 4.98 | 2.25 | 26.95 | 40.8 | 5.31 | 2.97 | 27.2 |
| 6 | Bis(O-isobutyldithiocarbonato) (2-amino-3-methylpyridine)copper(II) |
469.5 | 74 | Green | 40.16 | 4.96 | 5.31 | 26.75 | 40.89 | 5.53 | 5.96 | 27.26 |
| 7 | Bis(O-isobutyldithiocarbonato) (2-amino-4-methylpyridine)copper(II) |
469.5 | 74 | Green | 40.2 | 4.91 | 5.35 | 26.65 | 40.89 | 5.53 | 5.96 | 27.26 |
| 8 | Bis(O-isobutyldithiocarbonato) (2-amino-5-methylpyridine)copper(II) |
469.5 | 79 | Green | 40.24 | 4.9 | 5.2 | 26.72 | 40.89 | 5.53 | 5.96 | 27.26 |
Table 1: Analytical data of the adducts of Bis(O-Isobutyldithiocarbonato)Copper(II) with nitrogen donor ligands.
Physicochemical investigations
Molar conductance measurements: The molar conductance measurements were conducted on millimolar solutions of the adducts formed between bis(O-isobutyldithiocarbonato)copper(II). These measurements were performed in dimethylformamide, and the results indicate that the synthesized adducts exhibit a non-electrolytic nature [18]. The observed molar conductance values for all the adducts fall within the range of 10.84 to 24.63 ohm-1 mol-1 cm2. These recorded values are notably lower than the expected molar conductance values for any uni-univalent electrolytes in this particular solvent [19]. This discrepancy suggests that these complexes possess a neutral and non-ionic character. The specific molar conductance values for the adducts are provided in Table 2.
| Name of the adduct | Molar conductance (Ohm-1 mol-1 cm2) | Magnetic data | |
|---|---|---|---|
| µeff (B.M) | Temperature(K) | ||
| Bis(O-isobutyldithiocarbonato) (2-bromopyridine)copper(II) |
13.2 | 1.82 | 298 |
| Bis(O-isobutyldithiocarbonato) (3-bromopyridine)copper(II) |
12.83 | 1.85 | 298 |
| Bis(O-isobutyldithiocarbonato) (4-acetylpyridine)copper(II) |
14.76 | 1.87 | 298 |
| Bis(O-isobutyldithiocarbonato) (3-hydroxypyridine)copper(II) |
21.3 | 1.93 | 298 |
| Bis(O-isobutyldithiocarbonato) (2-methoxypyridine)copper(II) |
15.5 | 1.94 | 298 |
| Bis(O-isobutyldithiocarbonato) (2-amino-3-methylpyridine)copper(II) |
23.63 | 1.95 | 298 |
| Bis(O-isobutyldithiocarbonato) (2-amino-4-methylpyridine)copper(II) |
15.6 | 1.94 | 298 |
| Bis(O-isobutyldithiocarbonato) (2-amino-5-methylpyridine)copper(II) |
19.3 | 1.86 | 298 |
Table 2: Molar conductance and mgnetic data of the adducts of Bis(O-isobutyldithiocarbonato)Copper(ii) with nitrogen donor ligands.
Magnetic measurements: The adducts formed between bis(O-isobutyldithiocarbonato)copper(II) and various nitrogen donor ligands exhibit magnetic moments falling within the range of 1.81-1.92 B.M, as presented in Table 2.
These observed values correspond well with magnetic moment measurements observed in numerous square pyramidal complexes of copper(II). The recorded magnetic moment values surpass the spin-only magnetic moment values, indicating a significant orbital contribution and suggesting that the unpaired electron resides in the dx2-y2 orbital. Furthermore, these values also imply the absence of direct metal-metal interactions, as reflected by the lack of hyperfine splitting in the ESR spectra of the synthesized adducts [20].
Spectral studies
Infrared spectra: The infrared spectra of the adducts formed between bis(O-alkyldithiocarbonato)copper(II) and various nitrogen donor ligands, such as 2-bromopyridine, 3-bromopyridine, 4-acetylpyridine, 3-hydroxypyridine, 2- methoxypyridine, 2-amino-3-methylpyridine, 2-amino-4-methylpyridine, and 2-amino-5-methylpyridine, were recorded within the range of 4000-200 cm-1. Noteworthy infrared peaks, crucial for elucidating the geometry of the adducts, are presented in Tables 3.
| S. no | Name of the adducts | Formula | Ν (C-S) | νas (C-O-C) | νs (C-O-C) | ν (Cu-S) |
|---|---|---|---|---|---|---|
| 1 | Bis(O-isobutyldithiocarbonato) (2-bromopyridine)copper(II) | Cu(S2COC4H9)2(C5H4N.Br) | 1021 | 1211 | 1131 | 330 |
| 2 | Bis(O-isobutyldithiocarbonato)(3-bromopyridine)copper(II) | Cu(S2COC4H9)2(C5H4N.Br) | 1030 | 1220 | 1122 | 325 |
| 3 | Bis(O-isobutyldithiocarbonato) (4-acetylpyridine)copper(II) | Cu(S2COC4H9)2(C7H7NO) | 1023 | 1216 | 1130 | 335 |
| 4 | Bis(O-isobutyldithiocarbonato) (3-hyroxypyridine)copper(II) | Cu(S2COC4H9)2(C5H5NO) | 1029 | 1218 | 1128 | 320 |
| 5 | Bis(O-isobutyldithiocarbonato)(2-methoxypyridine)copper(II) | Cu(S2COC4H9)2(C6H7NO) | 1035 | 1209 | 1124 | 321 |
| 6 | Bis(O-isobutyldithiocarbonato) (2-amino-3-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 1032 | 1206 | 1128 | 337 |
| 7 | Bis(O-isobutyldithiocarbonato) (2-amino-4-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 1034 | 1204 | 1136 | 339 |
| 8 | Bis(O-isobutyldithiocarbonato)(2-amino-5-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 1028 | 1207 | 1140 | 353 |
Table 3: Important infrared bands (cm-1) of the adducts of bis(o-isobutyldithiocarbonato)copper(ii) with nitrogen donor ligands.
In the IR spectra of the adducts involving copper(II) dithiocarbonates and various nitrogen donors, distinct bands appear in the range of 1198-1220 cm-1 and 1137-1150 cm-1, corresponding to the asymmetric and symmetric stretching of the C-O-C group. The presence of a single band corresponding to the ν(C-S) stretching vibration suggests that xanthate acts as a symmetrical bidentate chelating ligand binding to the metal ion. Another band, observed with medium to strong intensity within the range of 320-352 cm-1, is attributed to the Cu-S stretching mode.
Comparing the infrared spectral data of the Cu((S2COR)2.L) adducts (where L represents the various ligands) with those of the free ligands reveals that the characteristic bands corresponding to the C-H out of plane bending vibrations of the free ligand exhibit a slight negative shift (5-10 cm-1 in their respective adducts. However, a notable positive shift is observed in characteristic vibrations such as ring C-C and C-N stretching vibrations, C-H in plane and ring in plane/out of plane stretching vibrations. In the case of adducts between bis(O-alkyldithiocarbonato)copper(II) and substituted pyridines, strong bands corresponding to C-N and C-C stretching vibrations are observed in the ranges of 1630-1580 cm-1 and 1470-1484 cm-1, respectively. In comparison to the free ligands, these bands exhibit a considerable positive shift due to extensive π bonding between the metal ion and the ligand. In the adducts involving 2-bromopyridine, the frequency at 614 cm-1 is attributed to C-Br stretching. Additionally, a strong band related to CBr stretching vibration is observed in the range of 600-650 cm-1 across all synthesized adducts. Similarly, for adducts involving bis(O-alkyldithiocarbonato)copper(II) and 2-methoxypyridine, the band at 3094 cm-1 is assigned to C-H stretching vibrations of the aromatic ring. The strong band at 1022 cm-1 in the free ligand corresponds to O-CH3 stretching mode, which shifts to a lower frequency upon coordination with the metal ion. The peak at 1608 cm-1 in the adduct with 3-hydroxypyridine is attributed to the stretching vibration of the C=N group in the pyridine ring. This peak shifts to higher frequencies, suggesting the formation of a coordination bond between the metal ion and the nitrogen atom of the heterocyclic ring. Complexes involving 3-hydroxypyridine also display a strong band corresponding to the ν(O-H) vibration. In adducts with 4-acetylpyridine, due to the presence of a CH3 group, the C-H stretching vibration of the methyl group occurs at lower frequencies than those of the aromatic ring. Across all adducts, a consistent observation is the considerable negative shift in the characteristic bands associated with C-H bending vibrations, confirming the coordination of ligands through their ring nitrogen to the metal ion.
This coordination of Lewis bases with metal ions has a more pronounced effect on C-N vibrations compared to C-C vibrations, ultimately establishing the coordination of nitrogen donors with the metal ion via ring nitrogen.
Electronic spectra: The electronic spectra of the adducts formed between copper(II) dithiocarbonates and the various nitrogen donor ligands were recorded in DMF within the range of 12500 cm-1 to 40000 cm-1). Copper(II), being a d9 ion, exhibits a single free ion term, 2D, which possesses a tenfold spin and orbital degeneracy. In this study, the prominent band associated with d-d transitions, commonly observed in many copper(II) complexes, appears within the range of 15500-17600 cm-1. The primary absorption band at around 16000 cm-1 can be attributed to dXZ, dYZ → dX2- Y2 transitions. A weaker shoulder accompanies this band due to the dz2 → dx2-y2 transition. A third transition, which is orbitally forbidden, merges with the broader band. The sequence of relative energy for these transitions is influenced by the extent of axial ligand-metal interaction. The presence of a single band with this intensity pattern indicates a square pyramidal geometry around the copper(II) ion in these 1:1 adducts. The adducts involving bis(Oisobutyldithiocarbonato) copper(II) display a broad band within the range of 15500-17000 cm-1, as presented in Table 4. The main absorption band around 16000 cm-1 corresponds to dXZ, dYZ → dX2-Y2 transitions. An intense band, possibly attributed to π-π* or n-π* transitions arising from coordinated ligands (xanthates), is also observed in the electronic spectra of the synthesized adducts, particularly in the region above 30000 cm-1.
| S. no | Name of the adduct | Formula | ν1(cm-1) dxz, dyzàdx2y2 | C-T Transitions (cm-1) |
| 1 | Bis(O-isobutyldithiocarbonato) (2-bromopyridine)copper(II) | Cu(S2COC4H9)2(C5H4N.Br) | 15691 | 34640 |
| 2 | Bis(O-isobutyldithiocarbonato)(3-bromopyridine)copper(II) | Cu(S2COC4H9)2(C5H4N.Br) | 15880 | 35180 |
| 3 | Bis(O-isobutyldithiocarbonato)(4-acetylpyridine)copper(II) | Cu(S2COC4H9)2(C7H7NO) | 15740 | 34300 |
| 4 | Bis(O-isobutyldithiocarbonato)(3-hyroxypyridine)copper(II) | Cu(S2COC4H9)2(C5H5NO) | 15540 | 35900 |
| 5 | Bis(O-isobutyldithiocarbonato)(2-methoxypyridine)copper(II) | Cu(S2COC4H9)2(C6H7NO) | 15941 | 34690 |
| 6 | Bis(O-isobutyldithiocarbonato)(2-amino-3-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 15633 | 35691 |
| 7 | Bis(O-isobutyldithiocarbonato)(2-amino-4-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 16012 | 38490 |
| 8 | Bis(O-isobutyldithiocarbonato)(2-amino-5-methylpyridine)copper(II) | Cu(S2COC4H9)2(C6H8N2) | 15990 | 37670 |
Table 4: Electronic spectral data of the adducts of bis(o-isobutyldithiocarbonato)copper(ii) with nitrogen donor ligands.
Mass spectral studies
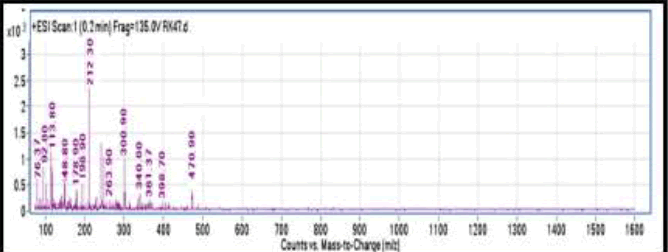
Mass spectra were recorded for the complex Cu(S2COC4H9)2.(2-methoxypyridine), synthesized in the present study. Mass spectrometry stands as one of the most essential methods for determining the molecular weight of a complex and identifying the fragments produced during bombardment. These fragments offer insights into the composition and properties of specific moieties within the complex. Potential formulae for the fragments and their corresponding m/z ratios are presented in Table 3. The mass spectra of the complex display a faint molecular ion peak at m/z 470.5. The presence of a molecular ion peak at this value suggests that the synthesized adducts exist in a monomeric form. In addition to the molecular ion peak, several other peaks corresponding to different m/z values are observed in the mass spectra of the complexes, as depicted in Figure 1. Peaks aligned with higher m/z values can be attributed to the direct fragmentation of the molecular ion, whereas those associated with lower m/z values may be interpreted as daughter fragments. The prominent peak for the complex appears at m/z 212.30, corresponding to the fragments (Cu(S2COC4H9)2) for the respective adducts. This finding indicates the formation of copper(I) xanthate as the most stable fragment within the mass spectral analysis of the adducts formed between bis(Oalkyldithiocarbonato) copper(II) and various nitrogen donor ligands.
Electron spin resonance spectral studies
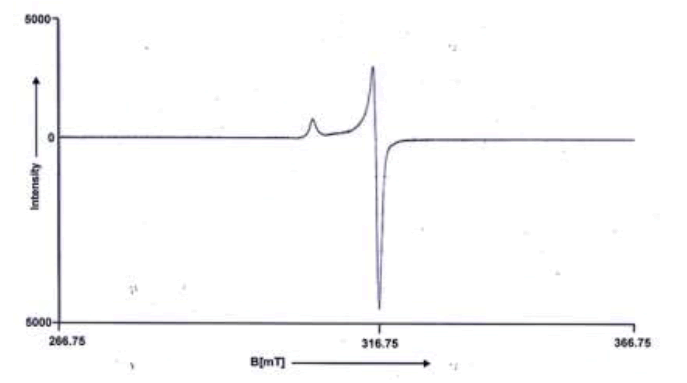
The ESR spectra of the Cu(II) adducts exhibit a resolution of parallel g? and g⊥ components, displaying an axial symmetry (g?=2.099, g⊥=2.024 for (Cu(S2COC3H7)2.C5H4N.Br). In this instance, g?>g⊥, indicating a slight elongation along one of the axes (z-axis), leading to anisotropy. The g values provide insight that the unpaired electron is localized in the dx2-y2 orbital, with the ground state being 2B1g. The confirmation of g?<2.3 underscores the covalent nature of the metal-ligand bond.
Upon comparing the obtained g values in this study to the g value of a free electron, 2.0023, it becomes evident that there is an enhanced covalent character in the bonding between the metal ion and the ligand molecule. This distinctive axial symmetry, featuring slightly varying g? and g⊥ values, aligns well with a penta-coordinated square pyramidal structure, as depicted in Figure 2.
Thermogravimetric analysis
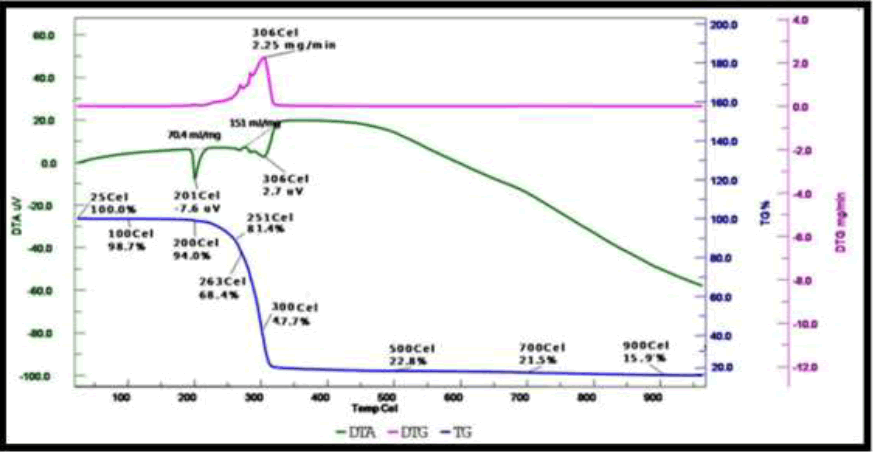
The adducts were subjected to TG analysis under a nitrogen atmosphere, spanning a temperature range of 25°C to 1000°C. The ultimate product observed in each adduct complex is a stable sulfide, CuS. In the thermal degradation of Cu(S2COC4H9)2(3-hydroxypyridine), the TGA curve exhibits an initial weight loss of 18.60% (calculated as 20.78%), attributed to the detachment of the 3-hydroxypyridine molecule. The subsequent weight loss at 52.30% (calculated as 53.35%) corresponds to the elimination of one xanthate molecule. With further heating, a weight loss of 84.10% (calculated as 85.9%) indicative of the loss of another xanthate moiety is observed. The final residual weight aligns with the formation of the stable sulfide, CuS, at 910°C (Figure 3).
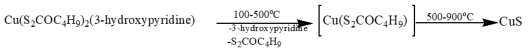
Antifungal activity
The in vitro biological screening effects of the investigated compounds were evaluated against the pathogen Fusarium oxysporium using the poisoned food technique on Potato Dextrose Agar (PDA) as the nutrient medium. The lipid membrane enveloping the cell selectively permits the passage of lipid-soluble substances, and lipophilicity plays a crucial role in controlling antimicrobial activity. Upon chelation, the polarity of the metal ion is notably reduced due to the orbital overlap of the ligand and the partial sharing of the metal ion's positive charge with the donor group. Both the parent compound, bis(O-alkyldithiocarbonato)copper(II), and their adducts with nitrogen donors exhibit antifungal activity. Interestingly, it has been observed that the adducts demonstrate a higher percentage of inhibition compared to the parent compound. As the concentration of the complexes is increased, the colony diameter of the fungus decreases, resulting in an increase in the percentage of inhibition.
The biological study results are depicted in Figure 3, and detailed data for these studies can be found in Table 5.
| S. no. | Addition complex | Colony diameter in control (mm) | Concenteration (ppm) | colony Diameter (mm) | % Inhibition I=((C-T)/C)×100 |
|---|---|---|---|---|---|
| 1 | Cu(S2COC4H9)2(3-bromopyridine) | 91 | 100 200 300 400 500 |
91.00 90.00 80.00 15.01 5.00 |
0 1.09 12.08 83.50 94.50 |
| 2 | Cu(S2COC4H9)2(2-methoxypyridine) | 91 | 100 200 300 400 500 |
88.50 64.70 50.70 15.90 5.00 |
2.74 28.90 44.28 82.52 94.50 |
| 3 | Cu(S2COC4H9)2(3-hydroxypyridine) | 91 | 100 200 300 400 500 |
91.00 86.00 81.50 78.50 5.00 |
0 5.49 10.43 13.73 94.50 |
| 4 | Cu(S2COC4H9)2(2-amino-5-methyl pyridine) | 91 | 100 200 300 400 500 |
90.00 76.00 24.00 17.00 5.00 |
1.09 16.48 73.62 81.31 94.50 |
Table 5: Antifungal activity of some bis(o-alkyldithiocarbonato)copper(ii) against Fusarium oxysporium colony diameter in control=91 mm.
Conclusion
In conclusion, dithiocarbamates, xanthates, and dithiocarboxylates are vital members of the 1,1-dithiolate family, drawing significant attention for their diverse applications and intriguing biological, structural, magnetic, electrochemical, and thermal activities. Xanthates are used as fungicides, pesticides, vulcanization accelerators, flotation agents, corrosion inhibitors, high-pressure lubricants, and HIV therapy agents, while both xanthates and thioxanthates are promising cancer inhibitors. Metallic xanthates are key in mineral flotation, cation separation, and preparing heterobimetallic sulfides. Sodium and potassium ethyl xanthates treat acute mercury poisoning, and transition metal xanthates have potential in nonlinear optics. Experimental methods synthesized potassium salts of Oalkyldithiocarbonates and prepared bis(O-alkyldithiocarbonato)copper(II) complexes. Using cupric chloride dihydrate, potassium salts of O-alkyl dithiocarbonates, and nitrogen donor ligands in acetone formed stable adducts, confirmed through various techniques. Adducts were prepared by mixing CuCl2·2H2O with the potassium salt of Oisobutyldithiocarbonate and substituted pyridines in acetone, yielding green precipitates. Elemental analysis, molar conductance, and spectroscopic studies confirmed adducts with a formula of Cu(S2COC4H9)2(L), where L represents various substituted pyridines. These adducts are insoluble in common solvents but soluble in DMSO and DMF. Physico-chemical investigations revealed 1:1 stoichiometry and a non-electrolytic nature. Magnetic measurements indicated unpaired electrons in the dx2-y2 orbital without metal-metal interactions. The adducts' air stability enhances their potential in antifungal agents, flotation agents, and nonlinear optics, highlighting the versatility and potential applications of these metal complexes.
References
- Solak S, et al. Chem Cent J. 2013;7:89.
[Crossref] [Google Scholar] [PubMed]
- Sajid A, et al. Asi J Chem. 2011;4:6.
- Santanaa J. et al. AIDS Res Hum Retrovir. 2012;4:71.
- Mellert W, et al. AIDS Res Hum Retrovir. 1988;4:71.
[Crossref]
- Rezaei BG, et al. J Serb Chem Soc. 2013;78(2):255-263.
- Wan D, et al. Macromol. 2005;38:10397-10405.
- Chang YK, et al. Chemosphere. 2003;52(6):1089-1094.
[Crossref] [Google Scholar] [PubMed]
- Carta F, et al. J Med Chem. 2013;56(11):4691-4700.
[Crossref] [Google Scholar] [PubMed]
- Rabhi A, et al. Thin Solid Films. 2009;517(7):2477-2480.
- Zelmon DE, et al. Mater Res Symp Proc. 1998;519:395.
- Sheldrick GM, et al. Acta Cryst. 2008;64(11):1179-1182.
[Crossref] [Google Scholar] [PubMed]
- Wagner CC, et al. Acta Farm Bonaerense. 2004;23:339-342.
- Gujarathi JR, et al. Der Pharm Chem. 2013;5(4):120-125.
- Wu G, et al. Mol. 2008;8:287.
- Chodurek E, et al. J Appl Biomed. 2013;11(3):173-185.
- Garje SS, et al. Coord Chem Rev. 2003;236(1-2):35-56.
- De Lima GM, et al. J Mol Struct. 2011;988(1-3):1-8.
- Sharma N, et al. Bull Chem Soc Jpn. 2011;84(8):855-861.
- Travnicek Z, et al. Trans Met Chem. 1999;24:633-637.
- Goel RK, et al. Indian J Phys. 1987;61:418.