Original Articles: 2023 Vol: 15 Issue: 5
Synthesis and characterization of Mg-Ni nanosized spinal ferrite
Vivek M Raut1, Sachin S Bangade1, Harshada S Gaikwad1, Shikhil S Wanjari2, Divya LChouhan3, Manish M Katiya3*
1Department of Chemistry, G. V. I. of Science and Humanties, Maharastra, India
2Department of Physics, Shri Mathuradas Mohota College of Science, Maharastra, India
3Department of Chemistry, Shri Mathuradas Mohota College of Science, Maharastra, India
- Corresponding Author:
- Manish M Katiya
Department of Chemistry,
Shri Mathuradas Mohota College of Science,
Maharastra,
India
Received: 07-Mar-2023, Manuscript No. JOCPR-23-91083; Editor assigned: 09-Mar-2023, PreQC No. JOCPR-23-91083 (PQ); Reviewed: 23-Mar-2023, QC No. JOCPR-23-91083; Revised: 10-May-2023, Manuscript No. JOCPR-23-91083 (R); Published: 17-May-2023, DOI:10.37532/0975-7384.2023.15(5).042.
Citation: Katiya MM, et al. 2023. Synthesis and Characterization of Mg-Ni Nanosized Spinal Ferrite. J. Chem Pharm.
Res., 15:042.
Copyright: © 2023 Katiya MM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
In this current work, the numerous goals are to urge prepared NiFe2O4 and Mg NiFe2O4 mineral primary solid solution nanoparticles through a basic, practical, and greener methodology and to boot do a nitty gritty characterization of NiFe2O4 and MgNiFe2O4 mineral primary solid solution nanoparticles. Along these lines, NiFe2O4 and Mg NiFe2O4 nanoparticles were prepared by an organic compound interceded sol-gel ignition strategy.
Keywords
Nanoparticles, Characterization, Nanocomposites, Organic compound, Methodology
Introduction
The nanoparticles embody the particles having size between one and one hundred nm. These particles have totally different properties at their atomic level because of their size. This modification in properties of nanoparticles is helpful in several fields. Engineering is one amongst the foremost fascinating fields for researchers since the last century. Numbers of developments are created since then within the field of engineering. Nanoparticles will be classified as metal nanoparticles, non-metal ceramic nanoparticles and semiconductor nanoparticles. Nanoparticles have those chemical and physical properties that make them terribly totally different from that of the corresponding bulk materials because of their tiny size and enormous surface to volume quantitative relation. They attract abundant attention as a result of their potential applications in several fields together with optics, electrics, magnetism, ceramics, and chemical change.
Spinel solid solution nanoparticles have received an excellent potential within the wide selection of applications like data storage, electronic devices to medical specialty, drug delivery, super capacitors, anode materials for lithium ion batteries, and microwave and radar absorbing material. Nickel solid solution (NiFe2O4) is one amongst the vital candidates among the mineral solid solution family as a result of its application in microwave, electronic, magnetic, and chemistry devices.
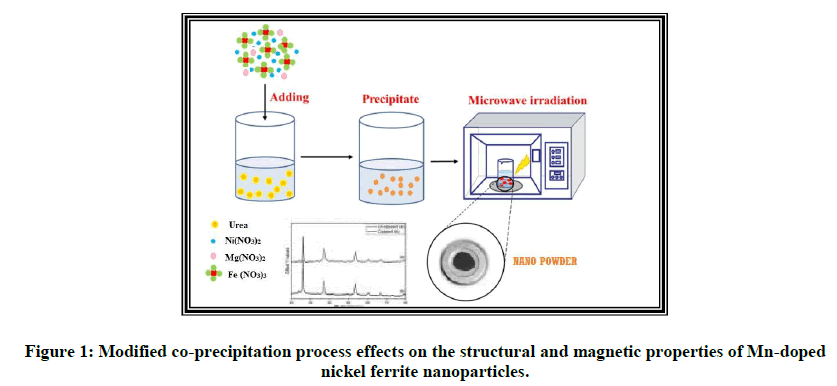
It exhibits an occasional coercivity, eddy and physical phenomenon loss; so, it's helpful for electronic devices like telecommunications and high frequency devices. Nickel and metallic element solid solution is extensively used in variety of electronic devices because of their high porousness at high frequency, exceptional high electrical electric resistance, mechanical hardness, chemical stability and affordable cost [1]. NiFe2O4 may be a documented inverse mineral with Ni2+ at octahedral (B-site) and Fe3+ ions distributed equally in tetrahedral (A-site) and octahedral sites (B-site) [2]. Metallic element solid solution (MgFe2O4) is one amongst the foremost vital ferrites. It a boxy structure of traditional spinel type and may be a soft magnetic n-type conductor, that finds variety of applications in heterogeneous chemical change, adsorption, sensors, and in magnetic technologies [3]. Recently, nanostructures of magnetic materials have received a lot of and a lot of attention because of their novel material properties that area unit considerably totally different from those of their bulk counterparts. It's been additionally detected that the electromagnetic radiation absorption capability of mineral solid solution nanoparticles depends on its structural characteristics like crystallinity, particle size, ion distribution, morphology, dopant ions, etc., consequently, goodish efforts are dedicated to prepare mineral solid solution nanoparticles by numerous chemical synthesis techniques like co-precipitation, hydrothermal/solvothermal methodology, microwave assisted synthesis, micro emulsion methodology, sonochemical synthesis, sol-gel combustion methodology, etc. (Figure 1) [4].
Among numerous chemical synthesis approaches, the sol-gel combustion methodology has numerous blessings like a speedy synthesis approach, formation of high purity product, unvaried composition and stabilization of stability phases, low energy consumption, and an easy, economic, and ascendible synthesis methodology [5].
Materials and Methods
In this gift work, the foremost objective is to arrange MgNiFe2O4 mineral solid solution nanoparticles via an easy, efficient and greener approach and to additional perform a close investigation on the impact of size of MgNiFe2O4 mineral solid solution nanoparticles. Therefore, MgNiFe2O4 nanoparticles were ready by organic compound from the microwave mediate combustion methodology. To the simplest of the authors data, this can be the primary report on the synthesis of MgNiFe2O4 nanoparticles by urea from the microwave mediate combustion methodology [6].
Experimental
Synthesis of Magnesium doped nickel ferrite: Magnesium doped nickel ferrite was synthesized by sol-gel microwave auto-combustion technique using urea as fuel. Magnesium nitrate, Nickel nitrate, iron nitrate and urea were weighed and mixed in double distilled water and mixed together [7]. The solution was stirred continuously and heated on magnetic stirrer at 80°C till a gel is obtained. The gel was then fired in a micro wave oven so that a floppy ash was obtained which then ground in a mortar pestle for four hours to get fine powder. This powder was then calcined at 800°C [8].
Synthesis of nickel ferrite: Nickel ferrite was synthesized by sol-gel microwave auto-combustion technique using urea as fuel [9]. Nickel nitrate, iron nitrate and urea were weighed and mixed in double distilled water and mixed together. The solution was stirred continuously and heated on magnetic stirrer at 80°C till a gel is obtained. The gel was then fired in a microwave oven so that a floppy ash was obtained which then ground in a mortar pestle for four hours to get fine powder [10]. This powder was then calcined at 800°C.
Results and Discussion
XRD Analysis
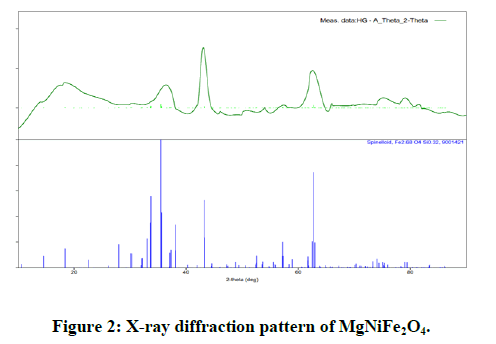
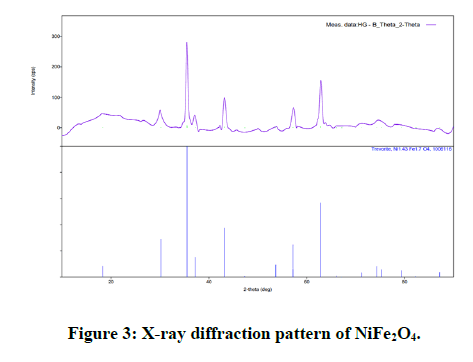
X-ray diffraction patterns of MgNiFe2O4 and NiFe2O4 one spinel ferrite nano particles are shown in Figures 2 and 3.
The XRD patterns well match all the characteristic reflections of cubic spinel structure without any extra peaks therefore the structure is single phase from the survey of literature, we found that the goodness values of our Rietveld refined sample have the good agreement with the reported values in previous literature. Figure 1 and 2 shows the typical Rietveld refined of X-ray pattern for sample x=1, and one respectively [11-17].
FTIR analysis
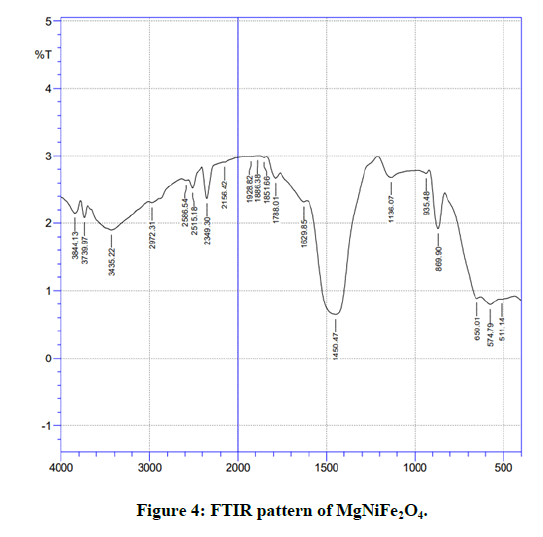
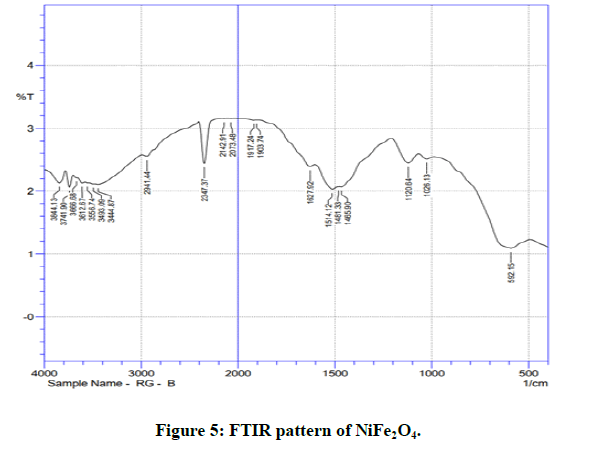
Fourier Transform Infrared (FTIR) spectra of the samples were recorded in the frequency range 400-4000 cm-1 at room temperature and are shown in the Figure 4 [18-21]. Two absorption frequency bands v1 and v2 are the characteristic bands of the spinel ferrites corresponding to stretching vibration of metal cation (Me) –oxygen anion (O2-) bond in tetrahedral and octahedral sites. The stretching vibrations of higher frequency band, v1 corresponds to tetrahedral site and stretching vibrations of lower frequency band v2 corresponds to octahedral site. Broad absorption peak around 3435.22 (MgNiFe2O4), 3493.03 (NiFe2O4) cm-1 and somewhat less broad absorption peak around1450.43 (MgNiFe2O4), 1514.12 (NiFe2O4) cm-1 in all the samples corresponds to stretching and bending vibration ofO-H interacting via H-bond expected due to absorbed water molecules on the surface (Figure 5) [22].
CONCLUSION
Magnesium substituted Nickel Ferrite, MgNiFe2O4 and MgNiFe2O4 were synthesized by sol-gel auto combustion technique with microwaves using urea as fuel. The super paramagnetic nanoparticles of size 11-23 nm was synthesized successfully by sol gel microwave assisted auto combustion method. The small coercivity and squareness ratio and non-saturation of the samples at even at high applied magnetic fields indicates that the most of the nanoparticles even if not all, are super paramagnetic in nature. The various properties of these nanoparticles studied indicates that they can be of potential applications use in many applied fields like MRI contrast agent, magnetic drug delivery and magnetic hyperthermia, data storage, sensors and catalysts.
References
- Yang C, Li W, Yang Z, et al. Nano Energy. 2015;18:12-19.
- Pan D, Wang S, Zhao B, et al. Chem Mater. 2009;21:3136-3142.
- Shao Y, Xiao J, Wang W, et al. Nano Letters. 2013;13:3909-3914.
[Crossref] [Google Scholar] [PubMed]
- Kaskhedikar NA, Maier J. Adv Mater. 2010;21:2664-2680.
[Crossref] [Google Scholar] [PubMed]
- Zhang J, Zhang Y, Wu X, et al. ACS Appl Mater Interfaces. 2018;10:42856-42864.
[Crossref] [Google Scholar] [PubMed]
- Shirsath SE, Liu X, Assadi MHN, et al. Nanoscale Horiz. 2019;4:434-444.
[Crossref] [Google Scholar] [PubMed]
- Manikandan V, Mirzaei A, Vigneselvan S, et al. ACS Omega. 2019;4:12919-12926.
[Crossref] [Google Scholar] [PubMed]
- Zhou ZH, Xue JM, Wang J, et al. Appl Phys. 2002;91:6015.
- Willey RJ, Noirclerc P, Busca G, et al. Chem Eng Commun. 1993;123:1.
- Choi S, Chung MH, Semin. Integr Med. 2003;53:1.
- Lai Z, Xu G, Zheng Y. Nanoscale Res Lett. 2007;2:40.
[Crossref] [Google Scholar] [PubMed]
- Corr SA, Rakovich YP, Gunko YK. Nanoscale Res Lett. 2008;3:87.
- Wang S, Zhou Y, Guan W, et al. Nanoscale Res Lett. 2008;3:289.
- Wu W, He Q, Jiang C. Nanoscale Res Lett. 2008;3:397.
[Crossref] [Google Scholar] [PubMed]
- Shirsath SE, Jadhav SS, Patange SM, et al. J Appl Phys. 2011;110:013914.
- Yang Y, Li M, Wu Y, et al. RSC Adv. 2016;6:25444-25448.
- Yan A, Liu X, Yi R, et al. J Phys Chem. 2008;112:8558-8563.
- Ayyappan S, Philip J, Raj B. J Phys Chem. 2009;113:590-596.
- Winiarska K, Szczygiel I, Klimkiewicz R. Ind Eng Chem Res. 2013;52:353-361.
- Liu C, Zou B, Rondinone AJ, et al. J Phys Chem. 2000;104:1141-1145.
- Toksha BG, Shirsath SE, Mane ML, et al. J Phys Chem. 2011;115:20905-20912.
- Reddy V. Superlattices Microstruct. 2015;82:165.