Original Articles: 2025 Vol: 17 Issue: 1
Studies on Exploring the Efficacy of Plant Based Silver Nanoparticles in Preventing Mercuric Toxicity on Blood
Mohit Pillai1, Nikita Patil1, Ashok A Shinde2, Raja Kumar Parabathina2*
1Department of Biotechnology, Institute of Biosciences and Technology, MGM University, Aurangabad, India
2Department of Biochemistry, Institute of Biosciences and Technology, MGM University, Aurangabad, India
- Corresponding Author:
- Raja Kumar Parabathina
Department of Biotechnology, Institute of Biosciences and Technology,
MGM University
Aurangabad,
India
Received: 05-Feb-2024, Manuscript No. JOCPR-24-126788; Editor assigned: 07-Feb-2024, PreQC No. JOCPR-24-126788 (PQ); Reviewed: 21-Feb-2024, QC No. JOCPR-24-126788; Revised: 05-Mar-2025, Manuscript No. JOCPR-24-126788 (R); Published: 12-Mar-2025, DOI:10.37532/0975-7384.2025.17(1).231.
Citation: Parabathina RK, et al. 2025. Studies on Exploring the Efficacy of Plant Based Silver Nanoparticles in Preventing Mercuric Toxicity on Blood. J. Chem. Pharm. Res., 17:231.
Copyright: © 2025 Parabathina RK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The research has a threefold objective: Synthesizing and characterizing AgNPs, investigating their impact on blood contaminated by mercury toxin, and examining AgNPs binding with bio-coated red blood cells.
Methods: This study explores the green synthesis of Silver Nanoparticles (AgNPs) using neem and Aloe vera leaves, characterized through UV-vis spectrophotometry and scanning electron microscopy.
Results: AgNPs couldn't prevent mercury-induced hemolysis, they effectively bound to RBCs in blood groups B+ and O+, with partial hemolysis in A+. These findings suggest potential biomedical applications.
Conclusion: This research contributes to the broader fields of nanotechnology, biotechnology, and environmental science, providing valuable insights into the potential of green-synthesized AgNPs and addressing the urgent need for innovative solutions to mercury contamination and its impact on human health and the environment.
Keywords
Silver Nanoparticles (AgNPs), Green synthesis, Mercury detoxification, Red blood cells, Aloe vera, Tulsi
Introduction
The synthesis of nanomaterials is plays one of the most active role in nanoscience. Special attention has been dedicated to nanomaterials that help improve the human quality life. A remarkable example is the Silver Nanoparticles (AgNPs) which are known by their inhibitory and bactericidal effects [1]. AgNPs can be produced with various sizes and shapes depending on the fabrication method which can be physical, chemical, biological and hybrid. The chemical methods are not friendly to the environment as they contain toxic elements, making them unsuitable for biomedical and health applications. Specifically, the widely used chemical reduction methods [2] usually employ toxic and perilous chemicals that are responsible for various biological risks. On the other hand, physical methods are expensive and incompatible with sizeable production of nanoparticles. Therefore, to avoid toxic and hazardous chemicals, the green synthesis methods have been developed, attracting significant interest because they are environment friendly, rapid, facile, and energy efficient [3]. Green synthesis using huge biological molecules derived from plant extracts [4] could facilitate size and morphology control of metal nanoparticles due to the presence of an innumerable quantity of biomolecules possessing bio-reduction and bio-stabilization ability [5].
Specifically, many plants have been used for silver nanoparticles synthesis, such as stem bark of Callicarpa maingayi, Terminalia cuneata, Illicium verum (star anise) and pod extract of Acacia nilotica [6-9]. Aloe vera extracts have been used for the synthesis of stable AgNPs in several previous articles investigating their antibacterial, antifungal, and mosquitocidal activity [10-13]. Aloe vera extracts have substances that lead to steric repulsion between individuals preventing nanoparticles from aggregation [14]. Using Aloe vera as surfactant prevents nuclei aggregation by decreasing the total surface energy because it contains a multitude of chemical constituents, such as amino acids, enzymes, minerals, vitamins (A, C and E), anthraquinones, lignin, monosaccharide, polysaccharides, salicylic acid, saponins, sterols, and minerals (calcium, phosphorous, potassium, iron, sodium, magnesium, manganese, copper, chromium and zinc) [15]. Population growth worldwide has led to increased demand for drinking water in recent years. However, water resources have suffered from pollution due to industrial sectors discharging various kinds of pollutants and harmful substances. Many kinds of pollutants such as herbicides, pesticides, dyes, plastics, oil derivatives and heavy metals [16-19] have been found in water effluents, which is the main reason behind the development of health issues among people. Mercury and its compounds are among the most harmful species reported and adversely affect human health [20]. Mercury affects the central nervous system, liver, and kidney. Further, it can disturb the immune system due to impaired hearing, vision, paralysis and emotional instability. Many analytical techniques are employed for detecting mercury ions such as inductively coupled plasma atomic emission and mass spectroscopy, cold vapor atomic absorption spectroscopy, cold vapor atomic fluorescence spectroscopy and high-performance liquid chromatography with UV-vis detection [21]. However, these techniques suffer from several limitations for instance time consumption, harmful solvents, equipment costs and complex procedures [22]. Therefore, developing new approaches to detecting and determining mercury ions becomes crucial and an urgent need. Mercury (Hg) is a heavy metal that is released into the earth’s atmosphere from natural sources such as volcanic action and human related sources such as coal combustion, waste combustion and smelting. Power plant flue gases are the largest source of Hg in the USA. In the atmosphere, chemical reactions convert Hg to divalent form (Hg2+) that can bind to particulate matter. Both Hg2+ and particulate bound Hg ingress from the atmosphere into water sources, where a small portion of the Hg can be converted into methylmercury by microorganisms and chemical processes. In water bodies, Hg accumulates in aquatic animals such as fish and others. The most common exposure of humans to mercury is through the consumption of marine and freshwater fish [23]. Historically, Hg has been used for medical purposes, such as in dental amalgam and in vaccines and flu shots as a preservative between 1930–1999 in the USA as well as in industrial products such as skin-lightening creams, antiseptic facial products, mercury-containing laxatives, diuretics, teething powders, and in latex paint. Mercury may also be ingested through high fructose corn syrup [24]. Mercury is harmless in insoluble form, but vapor or soluble forms such as inorganic mercury or methylmercury can be extremely toxic to humans. Most human mercury exposure occurs through inhalation of elemental mercury vapor released from dental amalgam and through the consumption of fish contaminated with methylmercury [25]. Once inhaled, elemental mercury vapor is rapidly accumulated into erythrocytes and undergoes oxidation to the mercuric ion (Hg) by catalase. Orally absorbed methylmercury is preferentially distributed into erythrocytes (~90%) and slowly turns into Hg through demethylation in the spleen and liver. It has been demonstrated that 6%-15% of total mercury in the blood of populations consuming high-fish diets exists in the form of inorganic mercury [26,27]. Because elemental mercury or methylmercury ultimately turns into Hg2+ in the body [28], mercuric salt has been commonly used to investigate the toxicity of mercury. After being ingested, Hg is absorbed into the bloodstream. Hg has been shown to affect protein stability, mineral loss and to result in enzyme denaturation. Silver Nanoparticles (AgNPs) are widely used for therapeutic interventions and diagnosis in medical practices and have gained increasing popularity as drug carriers, nano-probes, bio-imaging and labeling agents. Along with increasing application of AgNPs in nano-medicine. However, concerns are also escalating over its potential toxicity against human health [29]. Potential adverse effects of AgNPs against human health have been first illuminated at a concentration range of 25~500 μg/mL for 10~72 hrs duration in vitro, with respect to its cytotoxicity on human cell lines, Reactive Oxygen Species (ROS) generation, oxidative stress, cell-cycle arrest, pro-apoptotic effects and genotoxicity. AgNPs readily enters the systemic circulation. Accordingly, much attention has been paid to the potential cardiovascular toxicity of AgNPs due to an easy access of AgNPs to the tissues of cardiovascular system like blood cells, heart, and blood vessels. Previously, we demonstrated that AgNPs can activate platelets, which ultimately contributed to increased thrombosis. Thrombosis and embolism are serious life-threatening complications of various diseases with hyper coagulable states that include cancer [29], nursing home confinement, surgery, trauma and heavy metal intoxication. In addition to platelets, Red Blood Cells (RBCs), a major cellular component of blood can participate in venous thrombosis through facilitating coagulation cascade and clot formation by providing a site for the assembly of prothrombinase and tenase complexes. Externalization of Phosphatidylserine (PS) an anionic phospholipid to the outer leaflet of lipid bilayer is key in this process [29].
Materials and Methods
Synthesis and characterization of nanoparticles: Aloe vera based leaf extract- (Preparation of leaf extract) 15 g of inner leaf juice of Aloe vera leaves was heated at 80°C for two hours and then dried. It is then use for aqueous extract, using a ratio of 0.1:3, dry material to solvent. The resulting extracts use in all synthesis after being filtered by gravity [1].
Preparation of Aloe vera based silver nanoparticles: 101.92 mg of AgNO3 is added in 50 ml of distilled water. Add 50 ml of this solution to 30 ml of aqueous Aloe vera extract. The whole reaction is carried out in presence of air and constant and neutral pH. Stir mixture at temperature of 57°C during 3 hours and then heat 2°C/min to reach 80°C holding for 2 hours until obtaining solution with small suspended particles that could be removed by simple filtration (0.45 μm).
Neem based leaf extract (Preparation of leaf extract): Collected fresh Neem leaves and rinse thoroughly with distilled water to remove dirt. Spread rinsed leaves on a clean surface in well-ventilated area away from direct sunlight. Allowed the leaves to dry naturally in the shade until they become crispy. The dried leaves are grinded into a fine powder using mortar and pestle.
Measure 5 g of Tulsi powder and transfer it to a clean glass beaker. Add 100 ml of distilled water to the beaker containing the Tulsi leaf powder. Place the beaker a hot plate or stirrer and heat the mixture at 60-80°C temperature for 30 minutes. Then, cool the mixture to reach room temperature. Filtered the extract using a filter paper to separate the liquid extract from any solid residue.
Preparation of Neem leaves based silver nano particles: Prepared a silver nitrate solution by dissolving ad 1 mM (16.987 mg) silver nitrate in 100 ml D/W. Place it at magnetic stirrer, set temp at 60-70°C. Add leaf extract drop wise. (nearly 10 ml leaf extract) until it turns yellowish-brown color. Keep stirring continuously for 1-2 hours, to ensure complete reduction of silver ions and formation of silver nanoparticles. After the reaction allow AgNPs to settle at room temperature. The obtained AgNPs are purified and separated from others by centrifuge at 6000 rpm for 20 min in D/W (repeat 3 times). After centrifugation the pellet was washed and dried and prepared powder of it. Dried silver nanoparticles were used for characterization. UV-vis spectroscopy measures the absorption of light by nanoparticles. Silver nanoparticles exhibit a characteristic absorption peak in the UV-vis spectrum, known as the Surface Plasmon Resonance (SPR) peak. The SPR peak position and intensity provide information about the size and concentration of the nanoparticles.
Scanning Electron Microscopy (SEM): SEM is another imaging technique that provides surface morphology information of nanoparticles. It is particularly useful for analyzing larger nanoparticles and obtaining 3D images. Silver nanoparticles on blood contaminated by mercury toxin. Mercury chloride dissolved 0.2 gm of Mercury Chloride (HgCl2) in 10 ml of distilled water. Mixed a small percentage of mercury chloride solution with blood.
Test: Mixed a small percentage of synthesized silver nanoparticles with blood and added the mercury chloride solution to the blood. Performed staining and observed the structures of the blood cells under light microscope and analyzed the structures and checked the nanoparticle efficacy. Binding of bio-coated RBCs with silver nanoparticles, bio-coating of RBCs with PEG: Prepared PEG buffer by mixing 100 ml of PEG in 50 ml distilled water. Taken blood samples of different blood group; A+, B+ and O+. Centrifuged blood samples at 10000 rpm for 5 min. Discarded supernatant. Combined RBCs and PEG buffer solution in equal proportion (1:1). Incubated overnight at 4°C. Next day centrifuged RBCs with low speed to pellet them out. Performed blood staining to ensure there is no destruction of RBCs after bio-coating. Binding of bio-coated RBCs with silver nanoparticles: Added silver nanoparticles solution in bio-coated RBCs. Incubated RBCs overnight at 4°C. Next day performed blood staining of all the different blood groups and observed the structures of the blood cells under light microscope and analyzed the structures and checked the nanoparticle efficacy.
Results
Synthesis of nanoparticles
Aloe vera based silver nanoparticles: The Aloe vera extract which are extracted and dried are mixed with AgNO3 for 3 hrs for proper breakdown of metabolites and giving maximum exposure to mixing with silver nitrate solution, the resultant product formed was then separated using gravity filtration. A brownish orange solution was formed which confirms the result of nanoparticles are formed (Figure 1).
Figure 1: Aloe vera based synthesized nanoparticles: A brownish orange solution was formed which confirms the result of nanoparticles are formed.
Neem leaves based silver nanoparticles: The neem leaves extracts were mixed with AgNO3 for 3-6 hrs until a greenish slurry was formed. This slurry was then dried under a hot air oven for 5 min at 70-80°C. The dried extract was scraped out and stored in a reagent bottle. The dried extract so formed were black colored powder in nature (Figure 2).
Figure 2: Neem leaves based silver nanoparticles: The dried extract was scraped out and stored in a reagent bottle. The dried extract so formed were black colored powder in nature.
Characterization of the nanoparticles
UV-Spectrophotometry: Silver nanoparticles exhibit a characteristic absorption peak in the UV-Vis spectrum, known as the Surface Plasmon Resonance (SPR) peak. The SPR peak position and intensity provide information about the size and concentration of the nanoparticles. Peaks were visualized under Ultra violet wavelength (190-900 nm) at ranging around 300-370 nm.
Scanning electron microscopy: The nanoparticles formed was visualized under scanning electron microscope at 3000x the magnification. Nanoparticles were visualized majorly of rod shaped, and having size ranging up to 2-5 nanoscales (Figure 3).
Figure 3: Scanning electron microscope: Nanoparticles were visualized majorly of rod shaped, and having size ranging up to 2-5 nanoscales.
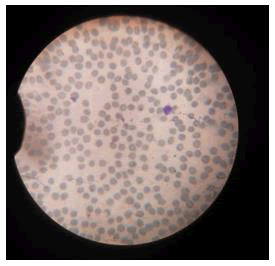
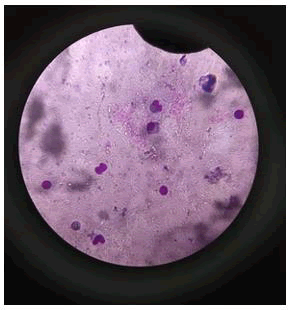
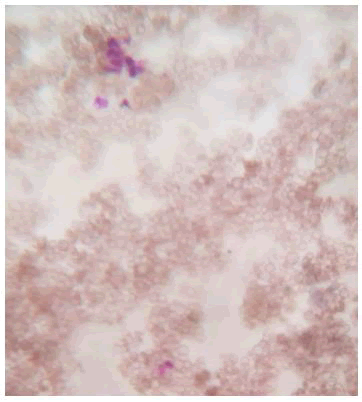
Action on blood: Impact of silver nanoparticles on blood contaminated by mercury toxin: For comparative study on blood, healthy blood cells were stained and visualized under microscope. Staining was performed using Giemsa stain and spherical shaped RBCs were observed along with other cells such as neutrophils and eosinophils (Figure 4).
Figure 4: Healthy blood cells were stained and visualized under microscope. Staining was performed using Giemsa stain and spherical shaped RBCs were observed along with other cells such as neutrophils and eosinophils.
Action of mercury on silver nanoparticle coated blood cells
Used 3 different blood groups i.e.: A+, B+ and O+.
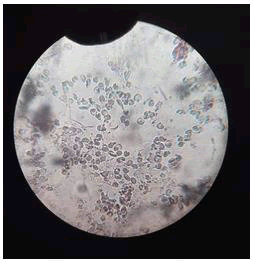
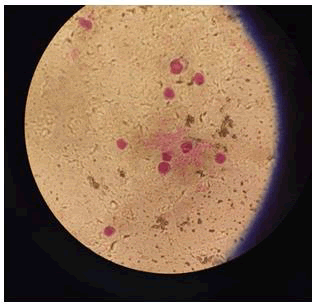
Control result: Mixed a small percentage of mercury chloride solution with blood, the result was negative which indicated total hemolysis of RBC cells (Table 1 and Figure 5).
| S. no | Test blood group | Shape analysis (without nanoparticles) | Result |
| 1 | A+ | Hemolysis total cell death | Negative |
| 2 | B+ | More than partial hemolysis | Negative |
| 3 | O+ | Hemolysis total cell death | Negative |
Table 1: Control analysis.
Figure 5: Control result (Hemolysis) The result were negative which indicated total hemolysis of RBC cells.
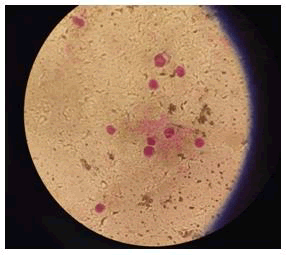
Test result: Mixed a small percentage of synthesized silver nanoparticles with blood and added the mercury chloride solution to the blood. The result was negative which indicated total hemolysis of RBC cells (Table 2 and Figure 6).
| S. no | Test blood group | Shape analysis (with nanoparticles) | Result |
| 1 | A+ | Hemolysis total cell death | Negative |
| 2 | B+ | More than partial hemolysis | Negative |
| 3 | O+ | Hemolysis total cell death | Negative |
Table 2: Test analysis.
Figure 6: Test result (Hemolysis) The result were negative which indicated total hemolysis of RBC cells.
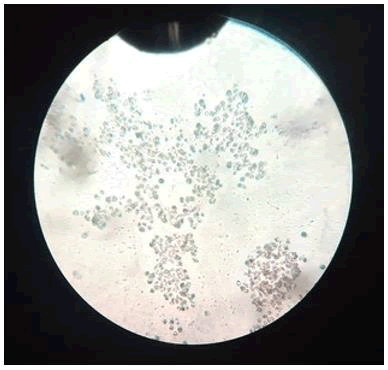
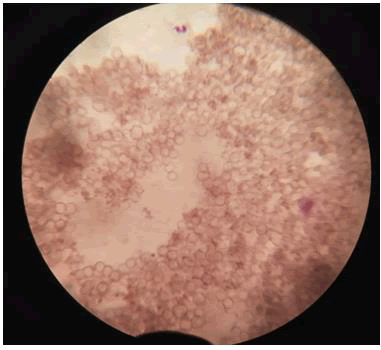
Binding of bio coated RBCs with silver nanoparticles: It is observed that when the RBCs were exposed to nanoparticles, cell hemolysis occurred at very high rates. This was due to the extreme small size of the nanoparticles which led to these particles to enter and cross the cell membrane, leading to cell membrane rupture and cause hemolysis (Figures 7-9).
Figure 7: A+ blood group (negative) (non-biocoated).
Figure 8: B+ blood group (negative) (non-biocoated).
Figure 9: O+ blood group (negative) (non-biocoated).
This hemolytic behavior was countered by creating a bio coat over the RBCs using bio coating agents i.e. PEG (Poly-Ethylene-Glycol). PEG coating was performed and then the cells were exposed to silver nanoparticles and observed under microscope.
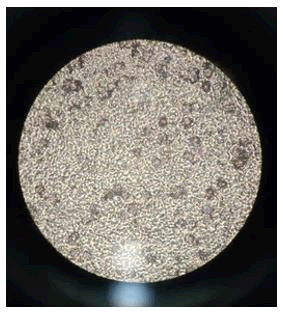
The results were observed in Table 3. This table indicates that out of three selected blood groups only B+ and O+ didn’t show hemolysis and A+ blood group shown partial hemolysis. It was observed that though there binding of nanoparticles over the PEG bio coat but in case of A+ blood group the nanoparticles crossed the membranes of RBCs and entered the cell leading to osmotic shock and cell death. However, B+ and O+ were alive which results in the binding of the nanoparticles over the surface of RBCs. This shows that the nanoparticles were prevented from entering the membrane walls of the cells (Figures 10-12).
| S. no | Test blood group | Shape analysis (with nanoparticles) | Result |
| 1 | A+ | Partial hemolysis | Negative |
| 2 | B+ | No hemolysis | Positive |
| 3 | O+ | No Hemolysis | Positive |
Table 3: Antibacterial activities of the Schiff base ligand and its polymeric complexes.
Through the above tabular data formed we can conclude that red blood cells undergone extreme oxidative stress in both the presence as well as absence of nanoparticles. Thus, without the help of any external bio coats, the oxidative action of mercury on the cell membranes could not be prevented under other circumstances. (The above test result resembles the action of silver-nanoparticles over non bio coated RBCs of blood group A+, B+ and O+).
• A+ blood group (negative) (non-biocoated).
• B+ blood group (negative) (non-biocoated).
• O+ blood group (negative) (non-biocoated).
Figure 10: A+ blood group (negative) (biocoated).
Figure 11: B+ blood group (positive) (biocoated).
Figure 12: O+ Blood group (positive) (biocoated).
Discussion
The green synthesis of Silver Nanoparticles (AgNPs) using plant extracts has demonstrated its potential as an environmentally friendly and efficient method. In this study, the use of Neem and Aloe vera leaves for AgNPs synthesis was explored. The characterization of these nanoparticles through UV-vis spectrophotometry and scanning electron microscopy confirmed their successful production and established their unique properties. One of the primary objectives of this research was to evaluate the impact of AgNPs on blood contaminated by mercury toxin. Notably, our experiments revealed that AgNPs were unable to prevent the destruction of Red Blood Cells (RBCs) induced by mercury chloride contamination. This result under schacores the severity of mercury toxicity and the urgent need for effective detoxification methods. As a response to the challenges posed by mercury-induced hemolysis, the focus of this study shifted to the binding of bio-coated RBCs with AgNPs. In these experiments, it was observed that AgNPs effectively bound to RBCs of blood groups B+ and O+, with only partial hemolysis observed in the A+ blood group. This finding points to the potential of AgNPs as a means of mitigating the effects of mercury toxicity, particularly in specific blood group scenarios.
Conclusion
In conclusion, the green synthesis of silver nanoparticles using plant extracts provides a promising avenue for the development of innovative nanomaterials with a range of applications. The significance of this research lies in its contribution to the understanding of mercury detoxification and the potential use of AgNPs as a therapeutic strategy. However, it is clear from our findings that while AgNPs exhibit favorable properties, they are not a complete solution for preventing mercury-induced hemolysis in all blood groups. Further research is necessary to explore more effective detoxification methods or to enhance the binding efficiency of AgNPs to RBCs. The pressing need for effective mercury detoxification methods, given the widespread contamination of water sources, underscores the importance of ongoing research in this field. This study opens the door to future investigations, including the optimization of nanomaterial binding, exploration of biomedical applications beyond detoxification, and further environmental remediation efforts. In conclusion, this research contributes to the broader fields of nanotechnology, biotechnology, and environmental science, providing valuable insights into the potential of green-synthesized AgNPs and addressing the urgent need for innovative solutions to mercury contamination and its impact on human health and the environment.
References
- Vélez E, et al. J Nanomater. 2018;2018(1):7215210.
- Bhui DK, et al. Carbohydr Polym. 2012;89(3):830-835.
[Crossref] [Google Scholar] [PubMed]
- Mohapatra B, et al. J Alloys Compd. 2015;637:119-126.
- Ahmed S, et al. J Adv Res. 2016;7(1):17-28.
[Crossref] [Google Scholar] [PubMed]
- Narayanan KB, et al. Eur J Plant Pathol. 2014;140:185-192.
- Shameli K, et al. Molecules. 2012;17(7):8506-8517.
[Crossref] [Google Scholar] [PubMed]
- Edison TN, et al. Spectrochim Acta A Mol Biomol Spectrosc. 2016;161:122-129.
[Crossref] [Google Scholar] [PubMed]
- Luna C, et al. Spectrochim Acta A Mol Biomol Spectrosc. 2015;141:43-50.
[Crossref] [Google Scholar] [PubMed]
- Jebakumar IE TN, et al. ACS Sustainable Chem Eng. 2013;1(10):1326-1332.
- Chandran SP, et al. Biotechnol Prog. 2006;22(2):577-583.
[Crossref] [Google Scholar] [PubMed]
- Medda S, et al. Applied Nanosci. 2015;5:875-880.
- Dinesh D, et al. Parasitol Res. 2015;114:1519-1529.
[Crossref] [Google Scholar] [PubMed]
- Zhang Y, et al. Nano Biomed Eng. 2010;2(4):252-257.
- Dang TM, et al. Adv Nat Sci Nanosci Nanotechnol. 2011;2(1):015009.
- Urvashi Nandal, et al. Int J Pharm Sci Rev Res. 2012;13(1):59-67.
- Alshawi JM, et al. ACS Omega. 2022;7(23):20405-20419.
[Crossref] [Google Scholar] [PubMed]
- Mohammed MQ, et al. Chemical Papers. 2022;76(2):715-729.
- Ali LA, et al. Int J Environ Sci Technol. 2021;18:2031-2050.
- Ismail HK, et al. J Polym Res. 2022;29(5):159.
- Pomal NC, et al. J Fluoresc. 2021;31:635-649.
[Crossref] [Google Scholar] [PubMed]
- Suvarapu LN, et al. Int J Anal Chem. 2017;2017(1):3624015.
[Crossref] [Google Scholar] [PubMed]
- He Z, et al. Materials. 2023;16(8):3083.
[Crossref] [Google Scholar] [PubMed]
- Guallar E, et al. N Engl J Med. 2002;347(22):1747-1754.
[Crossref] [Google Scholar] [PubMed]
- Kostyniak PJ. N Y State Dent J. 1998;64(4):40.
[Google Scholar] [PubMed]
- Lim KM, et al. Environ Health Perspect. 2010;118(7):928-935.
[Crossref] [Google Scholar] [PubMed]
- Berglund M, et al. Environ Health. 2005;4:1-1.
[Crossref] [Google Scholar] [PubMed]
- Rodrigues JL, et al. Talanta. 2010;80(3):1158-1163.
[Crossref] [Google Scholar] [PubMed]
- Zalups RK. Pharmacol Rev. 2000;52(1):113-144.
[Google Scholar] [PubMed]
- Bian Y, et al. Part Fibre Toxicol. 2019;16:1-4.
[Crossref] [Google Scholar] [PubMed]