Original Articles: 2023 Vol: 15 Issue: 2
Standardizing the assessment of amoebicidal efficacy of contact lens solutions against Acanthamoeba species
Lieke de Kroon1, Anna Clara Randag2, Henny Otten3, Barbara Schimmer4, Marlou Tehupeiory-Kooreman1, Cindy Arias Claro-Handgraaf1,5, Foekje Francina Stelma1*
1Department of Medical Microbiology, Radboudumc, Nijmegen, The Netherlands
2Department of Opthalmology, Rotterdam Ophthalmic Institute, Rotterdam, The Netherlands
3Department of Opthalmology, Visser Contactlenzen, Nijmegen, The Netherlands
4Department of Public Health, National Institute for Public Health and the Environment (RIVM), Bilthoven, The Netherlands
5Department of Medical Microbiology and Infectious Diseases, Canisius Wilhelmina Hospital (CWZ), Nijmegen, The Netherlands
- Corresponding Author:
- Foekje Francina Stelma
Department of Medical Microbiology
Radboudumc
Nijmegen
The Netherlands
E-mail: Foekje.Stelma@radboudumc.nl
Received: 01-Feb-2023, Manuscript No. JOCPR-22-81691; Editor assigned: 06-Feb-2023, PreQC No. JOCPR-22-81691(PQ); Reviewed: 20-Feb-2023, QC No. JOCPR-22-81691; Revised: 06-Mar-2023, Manuscript No. JOCPR-22-81691 (R); Published: 13-Mar-2023, DOI:10.37532/0975-7384.2022.15(2).017.
Abstract
To date no standardized methods are used in order to assess the amoebicidal efficacy of commercial contact lens solutions for both trophozoites and cysts of Acanthamoeba species. Here we present two methods that are suitable for this purpose: The Spearman-Karber log reduction method and XTT colorimetric assay. Acanthamoeba castellanii (ATCC 50370) and A. polyphaga (ATCC 30461) trophozoites were cultured in peptone-yeast extract-glucose medium. Cysts were developed in Neff’s encystement medium for 1 week. Spearman-Karber and XTT colorimetric assay were used to evaluate trophozoite and cystocidal efficacy of Multi-Purpose Contact Lens Solutions (MPS).With trophozoites, the Spearman-Karber method gave a log reduction estimate of morphological kill between log 0.83 and log 3.61 of the various contact lens solutions, enabling the differentiation between efficacious and less efficacious solutions. With cysts the maximum log reduction of 2.17 was achieved for all 3 MPS solutions at 8 hours. The XTT colorimetric assay showed reduction in trophozoite metabolic rates between 50 and 100% as provided by an optical density signal. All lens fluid solution with a reduction rate >90% showed residual growth of Acanthamoeba after one week of incubation on nutrient agar covered with Enterobacter aerogenes. Both methods give reproducible estimates of amoebicidal efficacy of contact lens solutions, however, XTT colorimetric assay should be followed by an assay for residual growth to test for viable cysts.
Keywords
Acanthamoeba Species; Lens Fluid Solutions; Efficacy; Colorimetric Assay; Log Reduction Method
Introduction
There has been an increase in the incidence of Acanthamoeba Keratitis (AK) associated with contact lens wear over the last decades [1,2]. AK is an infection of the cornea due to Acanthamoeba species and is associated with the use of soft Contact Lenses (CL). Personal hygiene is an important factor when using contact lenses and cleaning by rubbing and rinsing the lenses [3], and disinfecting them with all-in-one CL solutions, hydrogen peroxide or povidone iodine CL solutions is strongly advised [4,5]. Over the past decades the distribution of CLs and CL solutions has been made available also to general drugstores and internet. Therefore, the supervision of CL use has been shifting from specialized opticians to unsupervised use [6,7]. Possibly the increased availability and use of unsupervised CL is an important factor in the increase in AK patients seen by cornea specialists [2]. Risk factors like rinsing lenses and CL cases in tap water, swimming and / or while wearing CL and using multipurpose solution for cleaning the lenses have also been forwarded [8]. Further, as was reviewed by Bradley et al. it has been repeatedly shown that CL solutions are not efficient enough in the killing of Acanthamoeba species, especially not the cyst life stage [9]. Acanthamoeba are free-living amoeba that can be found everywhere, such as in tap water, surface waters, the air, soil and in vegetables. It consists of 23 genotypes (T1-T23), which are subdivided based on the nucleotide sequence of the 18S rRNA gene [10]. The most common genotype causing keratitis is T4. A. castellanii and A. polyphaga belong to this genotype [11]. The life cycle of Acanthamoeba consists of two stages: trophozoite and cyst. Trophozoites are the metabolically active stage in which the amoeba replicate and may infect individuals. Cysts are the metabolically inactive stage in which the trophozoite transforms to protect itself from adverse environmental conditions. The cyst stage has a double cell wall consisting of an ectocyst and endocyst, which is known to be much more resistant to disinfectants [12].
Before market release, CL solutions are tested according to an International standard protocol that describes European quality regulations, ISO 14729 [13]. However, this protocol only tests for bacteria and fungi killing. The protocol argues that testing for Acanthamoeba species is not essential as AK keratitis is very rare and a lens care system that eliminates contamination with bacteria will also reduce the incidence of Acanthamoeba contamination to a large extent. It has been argued that cysts, being the dormant stage, are not infectious. Therefore, inducing encystment by lens fluid solutions could be an effective preventive measure against AK. For assessing CL solution efficacy inducing Acanthamoeba trophozoite encystment, the protocol of trophozoite encystement was developed and described in the European quality regulations ISO 19045 [14]. This protocol does not describe a method to assess the efficacy of the disinfecting components in contact lens solutions against trophozoites and cysts of Acanthamoeba species, as it only aims to measure the encystment rate. However, after encystment Acanthamoeba cysts can still revert to trophozoites. Therefore also cysts should be considered an extra risk for AK development and contact lens solutions should be effective against both cyst and trophozoites, as has also been argued by others [15].
The present study describes two in-vitro assays suitable for assessing the amoebicidal efficacy of CL solutions. The Spearman-Karber method [16,17] is suitable for testing amoebicidal efficacy against both trophozoites and cysts of Acanthamoeba species. The XTT-colorimetric assay [18,19] followed by a residual growth assay, will test the amoebicidal efficacy against trophozoites and viable cysts.
Materials and Methods
Trophozoite and cyst culture
We selected two subtypes of Acanthamoeba species for this study as different subtypes may have various biological characteristics. A. castellanii (ATCC 50370)and A. polyphaga (ATCC 30461) trophozoites were grown in peptone-yeast extract-glucose (PYG) medium comprising: 0.98 g MgSO4⋅7 H2O L-1 ; 1 g C6H5Na3O7⋅2 H2O L-1; 0.02 g Fe(NH4)2(SO4)2⋅6 H2O L-1; 0.34 g KH2PO4 L-1; 0.355 g Na2HPO4⋅7 H2O L-1; 20 g proteose peptone L-1; 2 g yeast extract L-1; 18 gD (+)-glucose-monohydrate L-1 and 0.059 g CaCl2⋅2 H2O L-1 of deionized water. The pH was adjusted to 6.5 ± 0.2. Eight mL PYG medium was transferred to T25 tissue culture flasks, an aliquot of trophozoites was added and the flask was incubated at 28°C.
A. castellanii (ATCC 50370) and A. polyphaga (ATCC 30461) cysts were produced using Neff’s encystement (NEM) medium comprising: 7.46 g KCl L-1; 0.96 g MgSO4 L-1; 0.04 g 2-amino-2-methyL-1,3-propanediol L-1 of deionized water. The pH was adjusted to 7.4-7.8 and after 5 hours to 9.0 with 1 M NaOH. To produce cysts, the trophozoites were harvested at a concentration of 1 to 2 × 107 trophozoites per mL through centrifugation at 780 rcf for 5 minutes. The pellet was washed three times with ¼ Ringer’s solution (Thermo Fischer Scientific, Hampshire, IK) and afterwards inoculated into 8.0 mL of Neff encystment medium for 7 days at 28°C. Amoebic concentrations were estimated by counting trophozoite and cysts using Bürker Türk cell counting chambers (Faust Laborbedarf AG, Schaffhaussen, Germany).
Test solutions
In order to illustrate these two methods, a selection of three multipurpose CL solutions (MPS) was made from a more extensive study (2). The main biocidal components in each MPS were: MPS 1 (0.0001% PHMB), MPS 2 (0.00016% Alexidine, 0.0003% Polyquaternium) and MPS 3 (0.001% Polyhexanide). The recommended disinfection times (hereafter RDT) for MPS 1 and MPS 3 were 4 hours and that of MPS 2 was 6 hours. All the CL solutions bottles were previously unopened and used within two weeks after opening.
Spearman-Karber log reduction method
Spearman-Karber computations were used to determine the level of Acanthamoeba kill in relation to exposure to the different contact lens solutions. ¼ Ringer’s solution was used as reference (R = amoeba not exposed to CL solution) [16].
The procedures described hereafter were performed for both A. castellanii and A. polyphaga. 4.95 mL of each multi-purpose contact lens solution (MPS) or ¼ Ringer’s solution was transferred to a large Greiner tube. Fifty µl of 5 × 106 cells mL-1 trophozoites or cysts were added to each Greiner tube in order to obtain a final concentration of 5 × 104 amoebic cells mL-1. Tubes with MPS CL solution and amoeba were incubated 0, 4, 6 and 8 hours at 28°C before the tubes were mixed thoroughly by vortex. Half a mL of each Greiner tube was transferred to 4.5 mL Dey-Engley Neutralizing Broth (DENB) in order to neutralize the exposure to MPS, making the 0-hour tubes a Positive Control (PC).
Following, tenfold serial dilutions of each DENB tube were made with PYG medium (theoretical concentrations of 5 × 103, 5 × 102, 5 × 101 and 5 × 100 cells mL-1). Two hundred µl per well from the serial dilutions was transferred to a 96 wells plate in triplicate. Hundred-and-twenty µl per well of the positive control was also transferred together with 80 µl per well PYG medium. The 96 wells plates were incubated at 28°C, with the incubation period depending on the stage of the Acanthamoeba: 1 week for trophozoites and 2 weeks for cysts. After the incubation period, each well was assessed for growth by visual examination using a phase contrast microscope. The number of positive wells (wells with at least 2 amoeba growing) were counted and the log reduction of each test solution was calculated using the Spearman-Karber equation [16].
XTT colorimetric assay
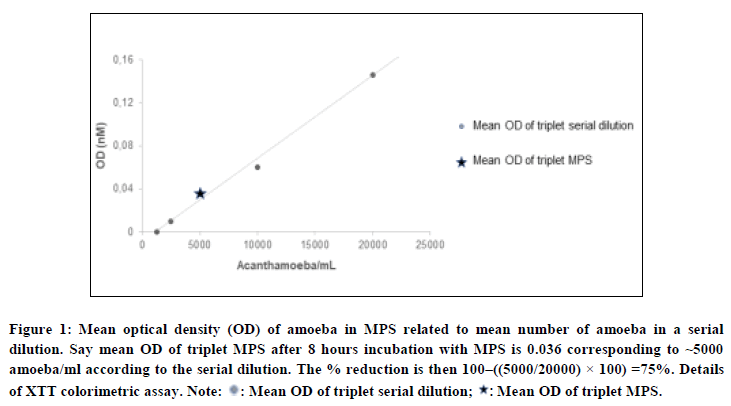
The procedure described hereafter was performed for both A. castellanii and A. polyphaga and repeated in order to have a beginning stock for each MPS. Trophozoites were seeded in a 96 wells plate in triplicate at 20,000 amoeba per well in 200 µl per well ¼ Ringer’s solution. A serial dilution of 20,000, 10,000, 5,000, 2,500 and 1,250 amoeba per well in Ringer’s solution was added in triplicate. The 96 wells plates were centrifuged, and the supernatant was aspirated and disposed (Table 1 and Figure 1) [18].
Hereafter 200 µl of each CL solution was added to each well with 20.000 amoeba. The wells with the serial dilution were filled with 200 µl of Ringer’s solution. The plates were then incubated for 4, 6 or 8 hours at 28°C. For A. castellanii 50 µl of XTT menadione reagent (end concentration 0.3 mg XTT mL-1 and 100 µM menadione) was immediately added to each well. For A. polyphaga the lens solution in each well was first aspirated, 200 µl Ringer’s solution was added and then 50 µl of the XTT menadione reagent was added. This was done because A. polyphaga needed a longer incubation period to observe the metabolic activity made visible by XTT. The plates for A. castellanii were incubated for 2 hours at 37°C and the plates for A. polyphaga were incubated for 24 hours at 37°C. Afterwards the XTT OD values were measured at 450 nm and 620 nm. All experiments were performed in triplicate for each of the incubation periods. Samples showing 90% reduction were transferred on nutrient agar with Enterobacter aerogenes (ATTC 13048) and incubated at 28°C for 2 weeks in order to control for residual growth.
This method shows the reduction in metabolic activity of trophozoites by a reduction in the Optical Density (OD) signal. For each experiment a reference serial dilution of number of amoeba/ml was incorporated. A fixed number of 10e4 amoeba/ml was incubated with each MPS.
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ |
| B | MQ | MPS1+ 10e4 Amoeb |
MPS2+ 10e4 Amoeb |
MPS3+ 10e4 Amoeb |
MPS 1 blanc | SD 2 × 104 |
SD 1 × 104 |
SD 5 × 102 |
SD 2× 102 5 × 102 |
SD 1 × 102 25 × 102 |
SD 6 × 102 5 × 102 |
MQ |
| C | MQ | MPS1+ 10e4 Amoeb |
MPS2+ 10e4 Amoeb |
MPS3+ 10e4 Amoeb |
MPS 2 blanc | SD 2 × 104 |
SD 1 × 104 |
SD 5 × 102 |
SD 2× 102 5 × 102 |
SD 1 × 102 25 × 102 |
SD 6 × 102 5 × 102 |
MQ |
| D | MQ | MPS1+ 10e4 Amoeb |
MPS2+ 10e4 Amoeb |
MPS3+ 10e4 Amoeb |
MPS 3 blanc | SD 2 × 104 |
SD 1 × 104 |
SD 5 × 102 |
SD 2× 102 5 × 102 |
SD 1 × 102 25 × 102 |
SD 6 × 102 5 × 102 |
MQ |
| E | MQ | MQ | MQ | MQ | MQ | RS | RS | RS | RS | RS | RS | MQ |
| F | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ |
| G | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ |
| H | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ | MQ |
|
Note: MQ: milliQ water; MPS: multipurpose solution with fixed amount amoeba (10e4); MPS blanco: MPS with no further additives; SD: serial dilution of amoeba; RS: Ringers solution 0,25%. |
||||||||||||
Table 1: Layout of 96 well plate for XTT assay.
Figure 1: Mean optical density (OD) of amoeba in MPS related to mean number of amoeba in a serial dilution. Say mean OD of triplet MPS after 8 hours incubation with MPS is 0.036 corresponding to ~5000 amoeba/ml according to the serial dilution. The % reduction is then 100–((5000/20000) × 100) =75%. Details of XTT colorimetric assay.
Note: ?: Mean OD of triplet serial dilution; ?: Mean OD of triplet MPS.
Statistical methods
The data was analysed using GraphPad Prism (Version 6.0; GraphPad software, Inc, San Diego). Differences were considered statistically significant at P ≤ 0.05. The data was analysed through one way ANOVA together with a Dunnett’s test. Amoebicidal efficacy was expressed as log reduction of growth when using the Spearman-Karber-method and percentage of reduction in optical density, when considering the XTT-colorimetric method.
Results and Discussion
Spearman-Karber method
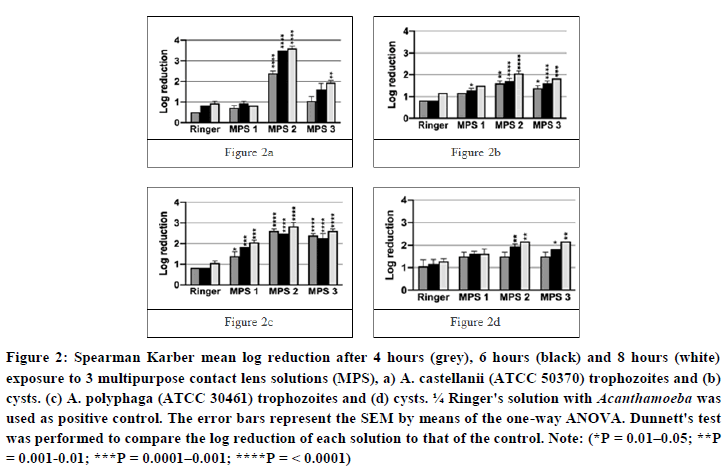
The efficacy against trophozoites (Figures 2a and 2b): MPS 1 and MPS 3 were relatively ineffective against A. castellanii trophozoites. At the Manufacturer’s Recommended Disinfection Time (MRDT), the log reduction of MPS 1 and 3 was not significant different from that of PC. After 8 hours, a maximum log reduction of 0.83 and 1.94 was achieved for MPS 1 and 3, respectively. MPS 2 did show a significant amoebicidal effect at MRDT when compared to PC, with a log reduction of 3.50. After 8 hours, this improved further to a log reduction of 3.61. For A. polyphaga trophozoites, each MPS were significant more amoebicidal than PC at MRDT, however none of the MPS exceeded a log reduction of 3.0. After 8 hours, the log reduction ranged between 2.06-2.83 for all three MPS. Note: a log reduction of 4.50 is the highest achievable as this corresponds to 100 % efficacy (=no growth).
The efficacy against cysts (Figures 2c and 2d): The log reduction after exposing Acanthamoeba cyst to the three MPS or ¼ Ringer’s solution, showed less variability. Considering A. castellanii cysts, the log reduction for any of the MPS did not surpass 2.17. MPS 1 showed no significant difference with PC at MRDT, and at 8 hours a maximum log reduction of 1.50 was achieved. Both MPS 2 and MPS 3 showed a significant better log reduction at MRDT when compared to PC (1.39 and 1.61 versus <1.0, respectively). After 8 hours, the maximum log reduction ranged between 1.83 and 2.06. Considering A. polyphaga cysts, the log reduction of MPS 1 and 3 at MRDT did not differ significantly with that of PC (1.50 and 1.94 versus 1.28 respectively). A maximum log reduction ranging between 1.61 and 2.17 was achieved for MPS 1 and 3 at 8 hours. Considering MPS 2, the log reduction was significantly better when compared to PC (1.94 and 1.39, respectively). A maximum log reduction of 2.17 was achieved for all 3 MPS solutions at 8 hours.
Figure 2: Spearman Karber mean log reduction after 4 hours (grey), 6 hours (black) and 8 hours (white) exposure to 3 multipurpose contact lens solutions (MPS), a) A. castellanii (ATCC 50370) trophozoites and (b) cysts. (c) A. polyphaga (ATCC 30461) trophozoites and (d) cysts. ¼ Ringer's solution with Acanthamoeba was used as positive control. The error bars represent the SEM by means of the one-way ANOVA. Dunnett's test was performed to compare the log reduction of each solution to that of the control.
Note: (*P = 0.01–0.05; **P = 0.001-0.01; ***P = 0.0001–0.001; ****P= <0.0001)
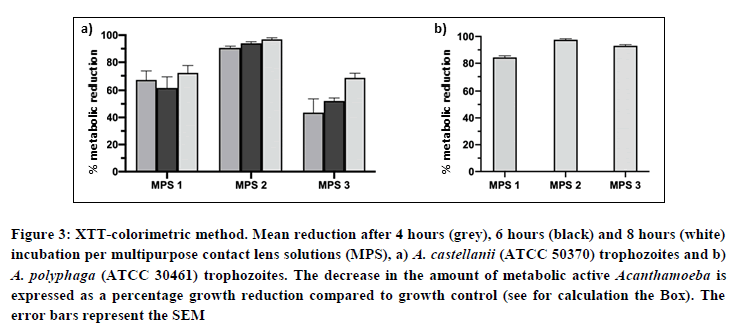
XTT colorimetric assay (Figures 3a and 3b): For A. castellanii, only MPS 2 showed a metabolic reduction >90% at MRDT and at 8 hours exposure to the lens fluid solution. For A. polyphaga, both MPS 2 and 3 showed a metabolic reduction >90% at 8 hours exposure to the lens fluid. The samples showing >90% reduction were plated on nutrient agar with E. aerogenes to test for viable cysts. All showed residual growth of trophozoites after one week of incubation.
Figure 3: XTT-colorimetric method. Mean reduction after 4 hours (grey), 6 hours (black) and 8 hours (white) incubation per multipurpose contact lens solutions (MPS), a) A. castellanii (ATCC 50370) trophozoites and b) A. polyphaga (ATCC 30461) trophozoites. The decrease in the amount of metabolic active Acanthamoeba is expressed as a percentage growth reduction compared to growth control (see for calculation the Box). The error bars represent the SEM.
Spearman-Karber log reduction and XTT colorimetric assay were performed to assess the amoebicidal efficacy of three contact lens solutions with varying amoebicidal efficacy against two different Acanthamoeba species. Figure 2 shows the results of the Spearman-Karber method and Figure 3 shows the results of XTTcolorimetric assay. The Spearman-Karber log reduction method provides a visual observation of the amoebicidal efficacy of each lens fluid. The XTT colorimetric assay provides information about reduction in metabolic activity which does in fact not reflect complete kill of trophozoites and cysts as cysts are metabolic inactive. Part of the reduction in metabolic activity can be explained by parasite encystation. We showed residual growth of amoeba even though reduction rates were >90%.
As the incidence of Acanthamoeba keratitis (AK) has increased over the past decades, the question came up once more, if CL solutions show sufficient amoebicidal effectivity. Quality control procedures, based on the ISO 14729 [13], describe how to examine lens fluid bactericidal and fungicidal efficacy. However, this protocol deliberately does not contain criteria regarding the effectiveness against Acanthamoeba species as there is no standard method of testing the efficacy of lens solutions against Acanthamoeba species and authorities have argued that if solutions are bactericidal, Acanthamoeba cannot grow as there are no bacteria to feed on. Still, in clinical laboratory practice we are increasingly confronted with Acanthamoeba contaminated contact lenses and storage cages over the past years, possibly due to decreasing professional supervised CL wear [6,7].
We developed a standardized protocol describing two in-vitro susceptibility assays which assesses the amoebicidal efficacy of contact lens solutions for both trophozoites and cysts. It was difficult to produce homogeneous results. Therefore, procedures were always performed in triplet taking continuous care of good mixing as amoeba tend to adhere to test tube surfaces causing variation in serial dilutions made [20]. Furthermore observations were subject to inter-observer variation. Therefore, when assessing the presence or absence of amoebic growth, we assumed that the presence of at least two trophozoites or cysts in the well, defined growth. The literature does not designate the criteria for a positive or negative assessment of the wells [4,21].
We observed a significant difference in lens fluid amoebicidal activity between the two subtypes tested in our experiment, A. castellanii and A. polyphaga. The variation between subspecies has also been described by others [4,17]. It seems that A. polyphaga shows less variation to the disinfecting effect of various CL solutions. This observation was made by Spearman-Karber method as well as by the colorimetric method. However, there may be a methodological bias as we had to modify the colorimetric protocol when testing A. polyphaga. The XTT incubation time in this assay was increased in experiments with A. polyphaga due to the slower metabolic rate of this subspecies. The reduction of XTT is based on the citric acid cycle. When a strain is less metabolically active, NADH is produced at a slower rate. Consequently, XTT will be converted less quickly. It therefore appears that diverse Acanthamoeba strains need different incubation times in the XTT colorimetric assay depending on their metabolic rate. No methodological adjustments were made in the Spearman-Karber method when testing different subtypes. This observation indicates that different Acanthamoeba species vary in their sensitivity to contact lens solutions, which, in itself, is an interesting observation as we do not know which subspecies is causing AK in the Netherlands.
Spearman-Karber method of susceptibility testing is a classical bioassay also described in virology and used in order to estimate EC50 and EC90 against virucidal and amoebicidal components elsewhere [16]. However, this method is laborious and subject to inter-observer variability. Therefore, we introduced the XTT colorimetric assay, a method that is more suitable for high-through-put testing. Although a trend to identical results may be present, the methods yielded different results. A reduction in OD of 90% (colorimetric assay) would theoretically correspond to a log reduction of 1.0 (Spearman-Karber method), an OD reduction of 99% corresponds to log reduction of 2.0, and an OD reduction of 99,9% corresponds to log reduction of 3.0. We observed higher efficacy using the Spearman-Karber method compared to the colorimetric assay. However, trophozoites and cysts were tested separately in the Spearman-Karber method, allowing higher efficacy measures in the experiments with trophozoites which are known from the literature [17], but lower efficacy in the experiments with cysts. The difference observed between the Spearman-Karber method and the XTT colorimetric assay probably reflects the difference in readout. Biologically these results may not be that different. The Spearman-Karber methods measures whether live Acanthamoeba are visible or not. The OD measured in the XTT colorimetric assay reflects amoebic metabolic activity. In other words, when trophozoites convert to the cyst form, they are metabolic inactive yielding a false negative signal in the XTT colorimetric assay [22]. By contrast, in the Spearman-Karber method we observed less amoebicidal efficacy concerning the cysts, which is in accordance with 90% reduction of OD in de colorimetric assay and that all samples showed residual growth in culture after one week. Therefore, the XTT colorimetric assay cannot be performed without a control for residual growth.
It is striking that in this “ISO-era”, no official criteria have been set for the minimum efficacy of contact lens solution against Acanthamoeba species [13]. In some studies, total killing of trophozoites or cysts is defined as a log reduction >3 [17,21,23]. Biologically this means that 99.9% of all Acanthamoeba are visually killed. Applying this criterion, only MPS 2 should be classified as an effective contact lens solution against A. castellanii trophozoites. Possibly the cut-off can be set at log 2 reduction (99% visual kill). However, it is unknown what proportion of survival is acceptable in real life. It is known for some time that MPS lens care solutions are less effective against Acanthamoeba. From the end of the 1980’ies and onwards there was a dramatic increase in cases of Acanthamoeba keratitis amongst contact lens users in the US [24]. In a retrospective case-control study this was associated to contact lens solution use [25]. This initiated a discussion on the need for testing of amoebicidal efficacy of lens fluid solution. Subsequently several studies showed that MPS solutions are not very effective against Acanthamoeba species[4,26]. This, in combination with laborious testing methods, made the FDA and commercial companies to come up with a strategy on how to assure safety against AK also by MPS. It was argued that AK is a very rare infection, depending on co-infection with a bacterial agent. Furthermore, breakthrough infections were most probable related to survival of resistant cyst-forms. So, if solutions were found to be bactericidal and if cyst formation could be prevented [14], the risk of AK due to inappropriate MPS solutions would be minimal. Further, a minimum 1-log reduction was suggested to indicate an appropriate amoebicidal activity [27]. On the other hand, Hiti et al. suggested that just one single surviving cyst can give rise to a new ‘‘amoeba population” [28]. As shown above, a 1-log reduction corresponding to a 90% reduction in a colorimetric assay, which in all our experiments resulted in residual amoebic growth after 1 week. Considering the above, studies in order to relate these cut-offs (1-log or 3-log) to clinical outcome are needed.
To date there are no studies using XTT colorimetric assay to test the efficacy of contact lens solutions. However, this assay could be valuable in high throughput settings. Other colorimetric assays like SRB, AlamarBlue and PrestoBlue with different seed volumes and different incubation times have been used in susceptibility experiments indicating that these methods should also be investigated further [29,30].
Conclusion
In conclusion, this study has shown that the Spearman-Karber method and the XTT colorimetric assay both yield quantitative results and can be used to evaluate the amoebicidal efficacy of contact lens solutions. The colorimetric assay should always be followed by a residual growth assay to test for viable cysts. We advocate that amoebicidal testing of lens fluid solutions should be integrated in quality control assessment of contact lens solutions, especially as to date, it is unclear what the infective dosage is of amoeba and its cyst forms.
Conflict of Interest
None of the authors have conflicting interests to disclose.
Funding
This project was part of a Bachelor thesis at the Radboudumc, Nijmegen, The Netherlands. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors. Authors performed the research within their institutions of employment.
Author’s Contribution
The research project was conceptualized, designed, and supervised by Fredrick CA. All experiments, results analysis along with manuscript draft was conducted by Hope NA. Chioma CJ and Gerald WU carried out results analysis and manuscript writing.
Lieke de Kroon was a bachelor student in laboratory medicine who performed most of the laboratory work for her bachelor thesis. She also sought solutions for technical issues, analysed the data and subsequently contributed substantially to the manuscript. Anna Clara Randag is a medical doctor trained in ophthalmology. She contributed substantially to the development of the protocol and the final manuscript. Henny Otten is a senior Optometrist who selected and provided the contact lens solutions and contributed to the manuscript. Barbara Schimmer is an epidemiologist who contributed substantially to the initial phase of design of the study. Cindy Arias Claro-Handgraaf and Marlou Tehupeiory-Kooreman are both senior technicians at the department of microbiology, Radboudumc. They respectively contributed by optimizing the protocols used and supervising the student. Foekje Francina Stelma is a medical doctor trained in microbiology and the senior scientist in this project, having the overall supervision of the research done, thereby contributing to the design of the study, overall supervision of the laboratory work and finally also contributing substantially to the manuscript.
Availability of Data and Materials
The datasets used can be accessed by doing a request to Foekje.Stelma@radboudumc.nl or A.Randag@oogziekenhuis.nl.
References
- Carnt N, Hoffman JM, Verma S, et al. Br J Ophthalmol. 2018;102(12):1621-1628. [Cross Ref][Google Scholar][Pub Med]
- Randag AC, Van Rooij J, Van Goor AT, et al. PLoS One. 2019;14(9):0222092. [Cross Ref][Google Scholar][Pub Med]
- Butcko V, McMahon TT, Joslin CE, et al. Eye Contact Lens. 2007;33(6 Pt 2):421-425. [Cross Ref][Google Scholar][Pub Med]
- Kobayashi T, Gibbon L,Mito T, et al. Jpn J Ophthalmol. 2011;55(5):547-557. [Cross Ref][Google Scholar][Pub Med]
- Sauer A, Meyer N, Bourcier T, et al. Eye Contact Lens. 2016;42(3):158-62. [Cross Ref][Google Scholar][Pub Med]
- de Lacerda AG, Lira M. Ophthalmic Physiol Opt. 2021;41(1):116-135. [Cross Ref][Google Scholar][Pub Med]
- Thite N, Desiato L, Shinde J, et al. Cont Lens Anterior Eye. 2021;44(6):101496. [Cross Ref][Google Scholar][Pub Med]
- Taher EE, Meabed EMH, Abdallah I, et al. J Infect Public Health. 2018;11(3):377-383. [Cross Ref][Google Scholar][Pub Med]
- Bradley CS, Sicks LA, Pucker AD. Clin Optom (Auckl). 2021;13:271-285. [Cross Ref][Google Scholar][Pub Med]
- Behera HS, Panda A, Satpathy G, et al. J Med Microbiol. 2016;65(5):370-376. [Cross Ref][Google Scholar][Pub Med]
- Behera HS, Satpathy G, Tripathi M. Parasit Vectors. 2016;9(1):442. [Cross Ref][Google Scholar][Pub Med]
- Moon EK, Lee S, Quan FS, et al. Exp Parasitol. 2018;188:102-106. [Cross Ref][Google Scholar][Pub Med]
- NEN-ISO. 14729. 2001.
- NEN-ISO. 19045. 2015.
- Lonnen J, Heaselgrave W, Nomachi M, et al. Eye Contact Lens. 2010;36(1):26-32. [Cross Ref][Google Scholar][Pub Med]
- Hamilton MA, Russo RC, RV Thurston. Environ Sci Technol. 1977;11(7): 714-719. [Cross Ref][Google Scholar]
- Kilvington S, Lam A. Invest Ophthalmol Vis Sci. 2013;54(7):4527-4537. [Cross Ref][Google Scholar][Pub Med]
- Hawser SP, Jessup C, Vitullo J, et al. J Clin Microbiol. 2001;39(7):2738-2741. [Cross Ref][Google Scholar][Pub Med]
- Gueudry J, Le Goff L, Compagnon P. J Antimicrob Chemother. 2018;73(7):1895-1898. [Cross Ref][Google Scholar][Pub Med]
- Mitsuwan W, S Sangkanu, Romyasamit C, et al. Int J Parasitol Drugs Drug Resist. 2020;14:218-229. [Cross Ref][Google Scholar][Pub Med]
- Beattie TK, Seal DV, Tomlinson A, et al. J Clin Microbiol. 2003;41(7):2992-3000. [Cross Ref][Google Scholar][Pub Med]
- McBride J, Ingram PR, Henriquez FL, et al. J Clin Microbiol. 2005;43(2):629-634. [Cross Ref][Google Scholar][Pub Med]
- Ustunturk M, Zeybek Z. Exp Parasitol. 2014;145:93-97. [Cross Ref][Google Scholar][Pub Med]
- Patel A, Hammersmith K. Curr Opin Ophthalmol. 2008;19(4):302-306. [Cross Ref][Google Scholar][Pub Med]
- Joslin CE, Tu EY, Shoff ME, et al. Am J Ophthalmol. 2007;144(2):169-180. [Cross Ref][Google Scholar][Pub Med]
- Kolar SSN, Manarang JC, Burns AR, et al. Cont Lens Anterior Eye. 2015;38(6):442-450. [Cross Ref][Google Scholar][Pub Med]
- Willcox M. Contact Lens update. 2009. [Google Scholar]
- Hiti K, Walochnik J, Schober EMH, et al. Cornea. 2006;25(4):423-427. [Cross Ref][Google Scholar][Pub Med]
- Vichai V, Kirtikara K. Nat Protoc. 2006;1(3):1112-1116. [Google Scholar]
- Fears AC, Metzinger RC, Killeen SZ. J Ophthalmic Inflamm Infect. 2018;24;8(1):19. [Cross Ref][Google Scholar][Pub Med]