Original Articles: 2025 Vol: 17 Issue: 1
Screening and Identification of Microbes in Different Milk Products
Ashwitha Kodaparthi*, Shaine Golla, Sravani Lavudya
Department of Chemistry, MNR Degree and PG College, Telangana, India
*Corresponding Author:
Received: 11-Sept-2023, Manuscript No. JOCPR-23-113366; Editor assigned: 14-Sept-2023, PreQC No. JOCPR-23-113366 (PQ); Reviewed: 28-Sept-2023, QC No. JOCPR-23-113366; Revised: 02-Jan-2025, Manuscript No. JOCPR-23-113366 (R); Published: 09-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).238.
Citation:Kodaparthi A, et al. 2025. Screening and Identification of Microbes in Different Milk Products. J Chem
Pharm Res., 17:238.
Copyright: © 2025 Kodaparthi A, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Milk is a fundamental and versatile dietary source, providing essential nutrients and serving as the primary food source for mammalian offspring. In addition to milk, many dairy products like butter, yoghurt, ghee, curd, cream, kefir, cheese, condensed milk, etc. have been produced and consumed around the globe. The screening and identification of bacteria and fungi in different milk products play a crucial role in ensuring their quality, safety, and shelf-life. This study aimed to characterize the microbial content of various milk products, including butter, yoghurt, curd, yakult, ghee, and milkmaid. he results revealed the presence of diverse bacterial and fungal species in each milk product. The most prevalent bacteria included Enterobacter sp., Bifidobacterium sp., Brevibacterium sp., Lactococcus sp., Providencia sp., Lactobacillus sp., Xenorhabdus sp., Proteus sp. Some are responsible for fermentation in yoghurt and curd whereas some milk products exhibited the presence of potential spoilage microorganisms, which might impact the products' quality and safety. Fungi, viz., Aspergillus, Mucor, Cladosporium, Rhizopus, and Yeast sp., were also identified in certain samples, highlighting the importance of monitoring fungal contamination in dairy products. Overall, this study provides valuable insights into the microbial composition of different milk products and emphasizes the significance of regular monitoring and quality control measures to ensure consumer safety and product integrity.
Keywords
Exploration, Characterization, Microbes, Milk, Milk products
Introduction
Since the earliest times, mankind has used the milk of goats, sheep, and cows as food. Today the term “milk” is synonymous with cow’s milk. The milk of other animals is spelled out, e.g. Sheep milk or goat milk when supplied commercially [1]. Goat’s milk is also consumed in some regions and is highly preferable in some parts of Europe, particularly in France and Italy, since the breeding of dairy sheep and goats is common there [2]. Milk is sterile at secretion in the udder but is contaminated by bacteria even before it leaves the udder. Except in the case of mastitis, the bacteria at this point are harmless and few further infection of the milk by microorganisms can take place during milking, handling, storage, and other pre-processing activities like feeding more starving and malnourished people in the developing world, treating people affected with cow’s milk allergies and gastrointestinal disorders, which is a significant segment in many populations of developed countries and filling the gastronomic needs of certain consumers, which is a growing market share in many developed countries [3-6]. The products which are prepared from milk are called dairy products. The category of milk and dairy products was divided into three main groups (i.e., milk, cheeses, yoghurts, milk drinks, and other dairy products) and seven sub-groups (i.e. whole milk, reduced fat milk, condensed and powdered milk, ripened and melted cheese, cottage cheese, and other dairy products) [7-10]. The bacteria can bring in beneficial effects or spoilage to milk. Some of the beneficial bacteria especially the lactic acid bacteria are called probiotics [11]. These probiotics are finding a better place in the nutraceuticals dairy and other food products which bring health benefits to the consumer like control of diarrhea, anti-hypertensive, hypercholesterolemia immunostimulatory, anticarcinogenic etc. [12]. Some bacteria are able to ferment lactose to lactic acid called homofermentative and with more end products called heterofermentative [13]. They are normally present in the milk and are also used as starter cultures in the production of cultured dairy products such as yogurt. Some examples in milk are Lactococci, L. delbrueckii subsp. lactis (Streptococcus lactis), Lactococcus lactis subsp. cremoris (Streptococcus cremoris), lactobacilli, Lactobacillus casei, L.delbrueckii subsp. Lactis. Other species present in milk includes strains of Bacillus, Clostridium, Cornebacterium, Arthrobacter, Lactobacillus, Microbacterium, Micrococcus and Streptococcus species can survive pasteurization and grow at refrigeration temperatures which can cause spoilage problems. Probiotics are live bacteria or yeasts that, when given in sufficient proportions, provide a health benefit to the host [14]. To date, with the growing interest in health consciousness, the concept of probiotic foods has gotten a lot of attention. A large number of probiotic species and strains belong to the genera Lactobacillus and Bifidobacterium [15]. Other yeast Saccharomyces are being used as probiotic microorganisms [16].
Some dairy products are rich in live beneficial microorganisms known as probiotics. The addition of probiotics enables dairy products to obtain unique quality characteristics and have health promoting effects. Probiotic dairy products satisfy people’s pursuit of health and are widely favored because of their easy absorption, high nutritional value, and various health benefits. However, its effectiveness and safety are still controversial [17].
Gram-positive lactic acid-producing bacteria that belong to the phylum Actinobacteria are Aerococcus, Micro bacterium, and Propionibacterium well as Bifidobacterium. Gram-positive bacteria that ferment carbohydrates into energy and lactic acid are members of the LAB family [18]. Depending on the organism, the metabolic processes are different if glucose is the major source of carbon: Homofermentative bacteria including Lactococcus and Streptococcus sp. give two lactates from a single glucose molecule, whereas glucose molecules are transformed into lactates with ethanol and carbondioxide (i.e., Leuconostoc, Lactobacillus delbrueckii sub sp. Bulgaricus, Streptococcus salivarius sub sp. Thermophiles). If bacteria are not available, then a spoonful of yogurt can also be used as it contains bacteria [19]. Probiotic bacteria like Streptococcus thermophilus, Lactobacillus acidophilus and bifidobacterium can also be used for the production of yogurt and it is commonly referred as bioyogurt [20,21].
Milk and dairy products are nutrient-dense foods providing 9.1% of the total energy supply. A high share (above 20%) in the supply of nutrients was noted in the case of calcium (54.7%), riboflavin (28.1%), vitamin B12 (26.1%) and phosphorous (24.6%). Dairy products are rich in nutrients that are essential for good bone health, including calcium, protein, vitamin D, potassium, and phosphorus. Adequate calcium intake influences skeletal calcium retention during growth and thus affects peak bone mass achieved in early adulthood.
High levels of calcium play an important role in the development, strength, and density of bones for children and in the prevention of bone loss and osteoporotic fractures in elderly people. Studies show that frequent consumption of dairy foods and milk should be recommended in order to prevent periodontal disease. Approaches for studying microorganisms in food have undoubtedly changed. Advances in molecular biology have provided more information on food associated bacteria, and have also provided the scientific community with sound, reliable and effective methods for detection, identification and typing of microorganisms from food.
The main interest of dairy microbiologists is to study the diversity and dynamics of microorganisms in dairy production and, possibly, to correlate the occurrence of certain microbial species and strains with desired flavor and sensorial traits of the products. Various molecular methods can be used depending on the level of information required by research. Microbiologists can be interested in identification, detection or typing of bacteria from a certain environment. Identification and detection can benefit from the availability of both culture-dependent and culture-independent techniques.
Materials And Materials
Sampling
A total of 6 different milk products in replicates were randomly collected from the following places tabulated in Table 1. The samples were put in sterile polyethene bags and transported to the laboratory for further analysis.
| S. no. | Food product | Image of food product | Place | Locality | State |
| 1 | Butter |  |
Vijetha supermarket | Nizampet | Telangana |
| 2 | Yoghurt |  |
Vijetha supermarket | Nizampet | Telangana |
| 3 | Curd |  |
Vijetha supermarket | Nizampet | Telangana |
| 4 | Yakult |  |
Ratnadeep | Nizampet | Telangana |
| 5 | Ghee |  |
Home made | Sakhinetipalli | Andhra Pradesh |
| 6 | Milkmaid |  |
Vijetha supermarket | Nizampet | Telangana |
Table 1: Showing locations of collection of milk and milk production.
Direct microscopic enumeration of milk products
After the physical examination and sterilization of the food products, the samples of each food product are taken and smears were prepared and observed microscopically for the detection of bacterial and fungal organisms. Generally, this microscopic identification is done by gram staining (for bacteria) and lactophenol cotton blue method (for fungi) and observing them under microscope at varying magnifications like 10X, 45X, 100X.
Isolation of micro organisms
Isolation of bacteria: The bacteria were isolated from spoiled fruits by using serial dilution agar plate method. The spoiled fruits were crushed into pre-sterilized mortar and pestle with distilled water to form suspension, which was serially diluted from 10-1 to 10-5 dilutions. 100 μl of different milk products’ suspensions from each dilution was spread over Nutrient Agar Medium (NAM) plates. The NAM was supplemented with amphotericin B (10 μg/ml) before pouring to prevent fungal growth and the inoculated Petri plates were incubated at 37°C for 24 hours for bacterial growth. After incubation the morphologically different colonies of bacteria were isolated and sub-cultured. The bacterial isolates were maintained and stored on NAM slants at 37 for further use.
The number of microbial cells in a sample that are viable, able to multiply under the controlled conditions per ml of sample is given in Colony Forming Unit (CFU).
Isolation of fungi: Isolation of fungi from each of the blemished fruit and vegetable was carried out using the method of the technique of Oyeleke and Manga. Spoiled tissues from the rotten fruits and vegetables were cut with a sterile scalpel and placed on the previously prepared Potato Dextrose Agar (PDA) in Petri dishes and incubated at 28°C for 3-4 days. The detected fungi were carefully isolated into pure cultures on potato dextrose agar plates.
Identification of bacterial isolates
The bacterial isolates were identified by cultural, morphological and biochemical characterization. These characterizations provide a preliminary understanding of the visual features of the bacterial isolates, which can help in identifying and categorizing different bacterial species.
Cultural and morphological identification: The isolated bacterial organisms were recognized based on morphological and biochemical characteristics according to the Bergey’s manual of systemic bacteriology. The morphology of the isolated bacterial cells was studied by various techniques like gram staining, capsular staining, endospore staining, flagellar staining and acid fast staining etc.
Biochemical characterization of bacterial isolates: The ability of microorganisms to use certain biomolecules and result in production of useful organic compounds for their use and forms the basis of many biochemical tests. The commonly used biochemical tests given below are performed on the bacterial isolates and observed for the result. The biochemical tests viz., indole test, gelatin hydrolysis test, catalase test, hydrogen sulfide test, nitrate reductase test, oxidase test, cellulase, lipase, methyl red test, Voges-proskauer test, citrate utilization test, amylase test, protease, carbohydrate fermentation test of sugars like D-glucose, trehalose, mannose, sucrose and lactose.
Identification of fungi: The incubated fungal plates are observed for the growth of fungi. Fungal staining is done with the lactophenol cotton blue method. According to this method the selected fungi is carefully removed from the plate using a sterile forceps or needle and place it on a clean glass slide. Add a drop of lactophenol cotton blue solution on the fungal smear and spread the fungal elements using a sterile needle. Now place a clean coverslip carefully on the prepared smear carefully and avoid the formation of air bubbles. Excess solution around the coverslip is cleaned with blotting paper. The smear is then observed under 10X or 45X magnification for the identification of fungi.
Graphical analysis
The data obtained was analyzed using MS-Excel 2019.
Results And Discussion
The study was performed to identify and isolate the various types of microorganisms in different milk products viz., yoghurt, butter, ghee, yakult, curd, and milkmaid procured from different supermarkets, Nizampet, Hyderabad. The findings showed that the products contain different types of microorganisms.
Following incubation, the unique bacterial colonies from each milk product viz., A1, B5, C3, D4, E6, F3, G3, H4, I6, J4, K6 and L2 were selected and used for further analysis. Many fungal colonies were developed of which the similar colonies were grown. The fungi after staining showed many types of fungal organisms of which the most commonly found isolates were labeled as F2, F3, F5, F7, F9, F10 and F12.
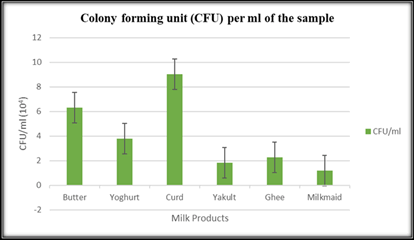
The data in the Table 2 represents the colony forming units per milliliter (CFU/ml) for various milk products. CFU/ml is a crucial measure used to assess the microbial content and quality of these dairy products. Among the tested milk products, curd exhibited the highest microbial count with 9.01 × 104 CFU/ml, followed by butter with 6.31 × 104 CFU/ml, and yoghurt with 3.78 ×104 CFU/ml. Other milk products, such as ghee and yakult, showed lower but still significant microbial counts with 2.26 × 104 CFU/ml and 1.82 ×104 CFU/ml, respectively. Milkmaid had the lowest microbial count among the products tested, with 1.171 ×104 CFU/ml. Monitoring CFU/ml is crucial for ensuring the safety and quality of dairy products, allowing manufacturers and regulatory bodies to implement appropriate measures to maintain product standards and prevent potential health risks associated with microbial growth (Table 2 and Figure 1).
| S. no | Milk product | CFU/ml |
| 1 | Butter | 6.31 × 104 |
| 2 | Yoghurt | 3.78 × 104 |
| 3 | Curd | 9.01 × 104 |
| 4 | Yakult | 1.82 × 104 |
| 5 | Ghee | 2.26 × 104 |
| 6 | Milkmaid | 1.171 × 104 |
Table 2: Colony Forming Unit (CFU) per ml of the sample.
Figure 1: Colony Forming Unit (CFU) per ml for different milk products.
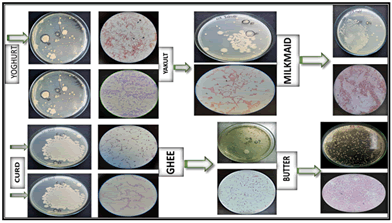
The Table 3 shows the cultural and staining characteristics of twelve bacterial isolates. The gram staining results provide crucial information about the cell wall structure of the bacterial isolates. K6, E6, A1, and G3 are Gram-negative (-), while L2, C3, D4, F3, B5, J4, and H4 are Gram-positive (Figure 2). Endospore staining indicates that none of the isolates exhibit endospores. Capsular staining shows that K6, L2, C3, D4, E6, and J4 possess capsules giving a positive capsular staining, whereas F3, B5, and H4 lack capsules (negative). K6, L2, I6, J4, G3, and H4 do not exhibit motility due to the absence of flagella and C3, D4, E6, F3, and A1 possess flagella and are motile. Acid Fast staining results reveal that K6, C3, E6, and A1 are acid fast staining negative (-). L2, D4, F3, and J4 show positive acid-Fast staining. B5, I6, G3 and H4 have variable acid-Fast staining results also display variable acid-Fast staining results (+/-).
| S. No | Cultural characters | Bacterial isolates | |||||||||||
| K6 | L2 | C3 | D4 | E6 | F3 | A1 | B5 | I6 | J4 | G3 | H4 | ||
| 1 | Growth medium | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 2 | Colony morphology | White | Pale white | White flat | White raised | Pale yellow | White uneven | Yellow translucent | White cottony | White flat | Translucent | Round yellow | White flat |
| 3 | Gram staining | - | + | + | + | - | + | - | + | - | + | - | + |
| 4 | Endospore staining | - | - | - | - | - | - | - | - | - | - | - | - |
| 5 | Capsular staining | + | + | + | + | + | - | + | - | + | + | + | + |
| 6 | Flagellar staining | + | + | - | - | - | - | - | - | + | + | + | + |
| 7 | Acid fast staining | - | + | - | + | - | + | + | - | +/- | + | +/- | - |
Table 3: Cultural and morphological characters for the bacterial isolates.
Figure 2: Cultural and microscopic observation of bacterial isolates.
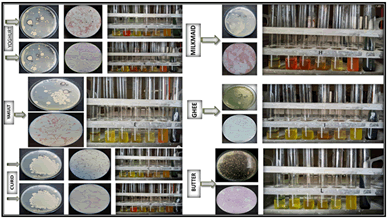
The Table 4 provided contains the results of different biochemical tests performed on bacterial isolates labeled K6, L2, C3, D4, E6, F3, A1, B5, I6, J4, G3, and H4. Among the isolates, K6 exhibited positive results for the methyl red, gelatin hydrolysis, catalase, and nitrate reductase. Isolate L2 showed positive reactions for the indole, cellulase, lipase, amylase, gelatin hydrolysis, and catalase. C3 displayed positive results for the Indole, MR, citrate utilization, cellulase, lipase, protease, and gelatin hydrolysis. Similarly, D4 showed positive reactions for the indole, mr, citrate utilization, cellulase, protease, amylase, gelatin hydrolysis, and catalase. E6 was positive for the cellulase, lipase, amylase, and nitrate reductase. F3 exhibited positive reactions for the citrate utilization, cellulase, gelatin hydrolysis, and catalase. A1 displayed positive results for the citrate utilization, cellulase, lipase, protease, amylase, gelatin hydrolysis, catalase, and nitrate reductase. B5 showed positive reactions for the MR, gelatin hydrolysis, and catalase. isolate i6 was positive for the cellulase, protease, amylase, gelatin hydrolysis, and catalase. J4 showed positive results for the cellulase, lipase, amylase test, gelatin hydrolysis, and catalase. G3 exhibited positive reactions for the lipase, protease, gelatin hydrolysis, catalase, and nitrate reductase. Lastly, H4 displayed positive results for the methyl red, voges-proskauer, citrate utilization, lipase, protease, amylase, gelatin hydrolysis, catalase, and nitrate reductase (Figure 3).
| S.No. | Biochemical test | K6 | L2 | C3 | D4 | E6 | F3 | A1 | B5 | I6 | J4 | G3 | H4 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | Indole test | - | - | + | + | + | - | - | - | - | - | - | - |
| 2 | Methyl red test | + | - | + | + | + | - | + | - | - | - | - | + |
| 3 | Voges-proskauer test | - | - | - | - | - | - | - | + | - | - | - | + |
| 4 | Citrate utilization test | - | - | + | - | - | - | + | + | - | - | - | + |
| 5 | Oxidase test | - | - | - | - | - | - | - | - | - | - | - | - |
| 6 | Cellulase | - | + | + | - | + | - | + | - | - | + | - | - |
| 7 | Lipase | + | + | + | + | + | + | - | + | - | + | - | - |
| 8 | Protease | - | + | + | + | - | - | + | + | + | - | + | - |
| 9 | Amylase test | + | + | + | - | + | - | + | + | + | + | + | - |
| 10 | Gelatin hydrolysis test | + | + | + | + | - | - | - | - | + | + | + | + |
| 11 | Catalase test | + | + | + | - | + | - | + | - | + | + | + | + |
| 12 | Hydrogen sulfide test | - | - | - | - | - | - | - | - | - | - | - | - |
| 13 | Nitrate reductase test | + | - | - | - | + | - | + | - | - | - | - | - |
| 14 | Carbohydrate fermentation test | ||||||||||||
| a) D-Glucose | ++/- | ++/- | --/- | ++/- | –/+ | ++/+ | ++/+ | ++/+ | ++/- | ++/+ | ++/+ | ++/+ | |
| b) Trehalose | ++/- | ++/- | –/- | ++/- | –/- | ++/- | ++/- | –/- | ++/- | ++/- | ++/- | ++/- | |
| c) Mannose | --/- | ++/- | –/- | ++/- | ++/- | ++/- | ++/+ | –/- | ++/- | ++/- | ++/- | ++/- | |
| d) Sucrose | ++/- | ++/- | –/- | ++/- | –/- | ++/- | ++/- | ++/- | ++/- | ++/- | ++/- | ++/+ | |
| e) Lactose | --/- | ++/- | –/- | ++/- | –/- | ++/- | ++/+ | ++/- | –/- | –/- | –/- | –/- | |
| Note: Acid a ++, Gas a + | |||||||||||||
Table 4: Biochemical characteristics of the bacterial isolates.
Figure 3: Biochemical tests for the bacterial isolates.
Carbohydrate fermentation test is used to determine the ability of bacteria to ferment specific carbohydrates, and the results are indicated by varying degrees of acid production and gas production during fermentation. Most of the isolates showed a positive gas and acid production for D-glucose, trehalose, mannose, sucrose and lactose.
By analyzing the results from the above tables, the bacterial isolates A1, B5, C3, D4, E6, F3, G3, H4, I6, J4, K6 and L2 were found to be Enterobacter sp., Bifidobacterium sp., Brevibacterium sp., Lactococcus sp., Providencia sp., Lactobacillus sp., Xenorhabdus sp., Proteus sp.Figure 4: Different types of fungi isolated from various milk product.
The fungal isolates F2, F3, F5, F7, F9, F10, and F12 were identified as different species within the genera Aspergillus, Mucor, Cladosporium, Rhizopus, and Yeast sp. respectively. These findings were obtained through the process of fungal isolation and subsequent identification based on morphological characteristics using Lacto phenol cotton blue method (Figure 4).
The identification of these bacterial and fungal isolates in different milk products emphasizes the need for proper quality control and hygiene practices to ensure the safety and integrity of dairy products.
Conclusion
In conclusion, the screening and identification of microbes in different milk products are essential steps in ensuring their quality, safety, and suitability for consumption. This study revealed the presence of diverse bacterial and fungal species in various milk products, including butter, yoghurt, curd, yakult, ghee, and milkmaid. Overall, this screening and identification process provide valuable insights into the microbial composition of different milk products, aiding in the development of strategies for product quality assurance. Regular monitoring of microbial content in milk products is vital to adhere to safety regulations, reduce health risks, and maintain consumer trust. Through effective quality control and hygiene practices, the dairy industry can continue to deliver safe, nutritious, and high-quality milk products to consumers worldwide.
Acknowdgement
The authors are grateful to Mr. M.N. Raju, Chairman, MNR Degree and PG College, Mr. M.S. Ravi Varma, Vice-Chairman, MNR Degree and PG College, Mr. M. Prasad, Director, MNR Degree and PG College, Kukatpally, Hyderabad for their constant support and encouragement.
Conflict Of Interest
The authors declare that there is no conflict of interest.
References
- Al?Zahrani MS. J Periodontol. 2006;77(2):289-294.
[Crossref] [Google Scholar] [PubMed]
- Bonjour JP. J Am Coll Nutr. 2005;24(sup6):526S-536S.
[Crossref] [Google Scholar] [PubMed]
- Caplice E, et al. Int J Food Microbiol. 1999;50(1-2):131-149.
[Crossref] [Google Scholar] [PubMed]
- Claus D. Berger’s manual of systematic bacteriology. 1986;2:1105-1139.
- Coppola S, et al. Dairy products. Molecular techniques in the microbial ecology of fermented foods. Springer. 2008;31-90.
- Fesseha H. Open J Vet Med. 2019;4(2):69-76.
- Fooks LJ, et al. Int Dairy J. 1999;9(1):53-61.
- Gareau MG, et al. Nat Rev Gastroenterol Hepatol. 2010;7(9):503-514.
[Crossref] [Google Scholar] [PubMed]
- Gibson GR, et al. J Nutr. 2000;130(2):391S-395S.
[Crossref] [Google Scholar] [PubMed]
- Haenlein GFW. Small Ruminant Res. 2004;51(2):155-163.
- He J, et al. Crit Rev Food Sci Nutr. 2022;63(32):1-20.
[Crossref] [Google Scholar] [PubMed]
- Heitmann BL. Nutrients. 2012;4(9):1219-1229.
[Crossref] [Google Scholar] [PubMed]
- Holzapfel WH, et al. Am J Clin Nutr. 2001;73(2):365s-373s.
[Crossref] [Google Scholar] [PubMed]
- Jay JM, et al. Food Microbiol. 1995:131-148.
- Joint FAO. World Health Organisation. London, Ontario, Canada. 2002;30(1):16-22.
- Kuipers OP, et al. Res Microbiol. 2000;151(10):815-822.
[Crossref] [Google Scholar] [PubMed]
- Lee K, et al. Nutrients. 2019;11(5):1035.
[Crossref] [Google Scholar] [PubMed]
- Quiles JL, et al. Studies on Periodontal Disease. 2013:251-278.
- Rizzoli R. Am J Clin Nutr. 2014;99(5):1256S-1262S.
[Crossref] [Google Scholar] [PubMed]
- Velez MP, et al. J Appl Microbiol. 2007;103(3):666-674.
[Crossref] [Google Scholar] [PubMed]
- Zhu K, et al. Clin Biochem. 2012;45(12):936-942.
[Crossref] [Google Scholar] [PubMed]