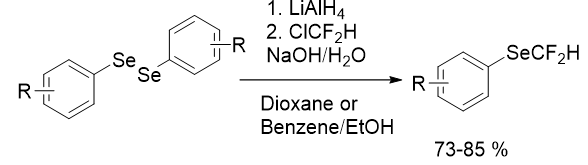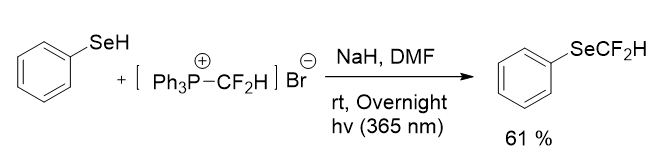Original Articles: 2025 Vol: 17 Issue: 1
Recent Advances in Difluoro, Monofluoromethylselenolation and (CF2HTe) Reactions
Yasir Mumtaz*, Jahangir Khan
Department of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, China
*Corresponding Author:
- Yasir Mumtaz
Department of Chemical Engineering, Nanjing University of Science and Technology, Nanjing, China
Received: 09-Sept-2024, Manuscript No. JOCPR-24-147636; Editor assigned: 11-Sept-2024, PreQC No. JOCPR-24-147636 (PQ); Reviewed: 25-Sept-2024, QC No. JOCPR-24-147636; Revised: 08-Jan-2025, Manuscript No. JOCPR-24-147636 (R); Published: 15-Jan-2025, DOI:10.37532/0975-7384.2024.17(1).239.
Abstract
Substituting functional groups with monofluoromethylseleno, difluoromethylseleno, and difluoromethyltelluro moieties has emerged as strategy to enhance lipophilicity and electron-withdrawing properties of compounds, thereby advancing their bioavailability for drug targets. These substitutions, denoted as -CFH2Se, -CF2HSe, and -CF2HTe, have demonstrated efficacy in medicinal chemistry, drawing considerable attention to difluoromethylselenolation and monofluoromethylselenolation techniques. In medicinal chemistry, where drug efficacy often hinges on compound's ability to interact with biological systems, enhancing lipophilicity and electron-withdrawing properties can improve drug's effectiveness. The introduction of selenium and tellurium-containing moieties represents novel approach in this pursuit. Two primary strategies have been explored: Indirect approaches involving the difluoromethylation of selenylated substrates and direct difluoromethylselenolation. The former requires initial synthesis of selenylated compounds, presenting significant drawback. In contrast, direct difluoromethylselenolation offers more streamlined process, bypassing need for pre-functionalization of substrates. This review focuses on recent developments in difluoromethylselenolation and difluormethyltelluration, highlighting challenges and advancements in utilizing benign difluoromethylselenylated reagents. Despite hurdles, pursuit of difluoromethylselenolation remains notable in medicinal chemistry research. Additionally, the article summarizes current progress in monofluoromethylselenolation reactions, providing insights into ongoing exploration of selenium-containing functional groups for drug development purposes. Through these discussions, the review aims to contribute to understanding and advancement of selenium-based methodologies in medicinal chemistry.
Keywords
Difluoromethylselenolation; Monofluoromethylselenolation; Difluormethyltelluration; Fluorination; Tellurium; Synthetic methods
Introduction
Monofluoromethylthio (SCFH2) and Difluoromethylthio (SCF2H) fluoroalkylthio groups are biologically active and have completely distinct physical and chemical properties than other organic compounds [1]. It's not only because it integrates two contributing components (sulfur and fluorine) into a single function, but also because of its unique features [2].
• SCF2H and SCFH2 have intermediate lipophilicity,
• Giving pharmaceutical chemists more options in the design of therapeutic candidates and potentially increasing the drug molecules' metabolic stability.
• Has an acidic proton, which makes it a weak hydrogen bond donor and also has electron-withdrawing characteristics [3].
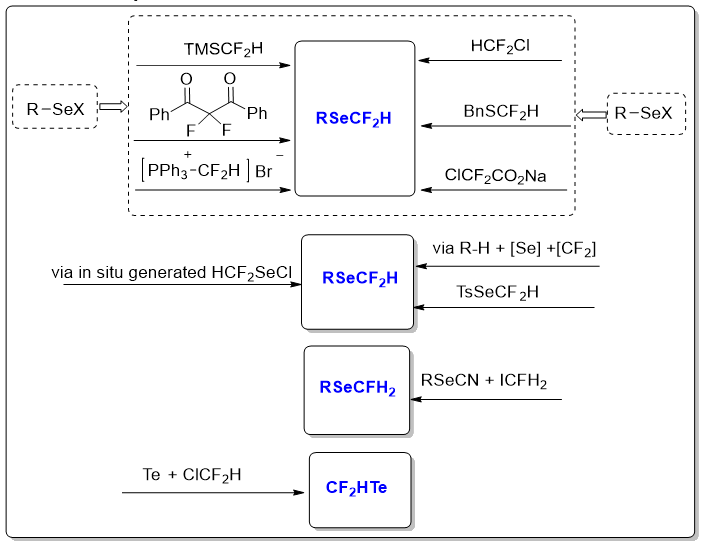
1-2 Difluoromethylsulfides (SCF2H) and Monofluoromethylsulfides (SCFH2) that can be used in a variety of late-stage investigations to change the functionality and bioactivity of the host molecules [4]. The pharma industry extensively uses both monofluoromethylthio and difluoromethylthio groups in numerous drugs and agrochemicals. Pyriprole (pesticide), SSH-108 (herbicide), nifedipin analogue, thymol analogues of sulindac, and cathepsin K inhibitors are some well-known examples of SeCF2H and SeCFH2 containing agrochemicals and drugs [5]. In the chalcogen family, the selenium atom has the same characteristics as sulfur [6]. The uncommon features of selenium coupled with oxygen and sulfur when involved in chemical bonding and complexes have sparked a flurry of research in domains like nanomaterials, organic synthesis, catalysis, and biology [7]. Organoselenium compounds exhibit antifungal, anticancer, and antiviral properties, and have been studied for the treatment of Alzheimer's disease [8]. Some selenylated compounds can be used in medication development, as evidenced by the medicine Ebselen, which has anti-oxidant, anti-inflammatory, and cytoprotective properties [9]. When compared to a fluoroalkylthio group, fluoroalkylseleno groups with more selenium atoms can provide stable, superiors, and functional groups with high lipophilicity [10]. However, unlike Sulphur, the relationship between fluorine and the next chalcogen (selenium) has received less attention [11]. Selenylated chemicals, on the other hand, are becoming increasingly important in a variety of domains, ranging from biological sciences to materials research [12]. As previously indicated, the relationship between fluorine and selenium has received less attention than that of sulfur. However, Difluoromethylselenides (SeCF2H) have a wide range of uses as Difluoromethylsulfides (SCF2H) [13]. Because some difluoromethylselenolated substituents have been recognized as valuable therapeutic functions, the Difluoromethylseleno group (HCF2Se) has recently attracted great attention [14]. As a result, tremendous progress has been achieved in developing efficient techniques for introducing the HCF2Se into organic compounds [15]. However, this paper is the first to provide a full overview of the numerous synthetic methods for SeCF2H, SeCFH2, and TeCF2H-containing compounds, as well as their distinctive uses [16]. This review covers all approaches that have been documented in the literature up to the end of 2024 [17]. Different practices were used on compounds comprising SeCF2H, TeCF2H, and SeCFH2. As the Billiard group has published a detailed study of SeCF3, we will concentrate on SeCF2H, SeCFH2, and TeCF2H (Figure 1).
Figure 1: Advances in Difluoro, Monofluoromethylselenolation and (CF2HTe) reactions.
Materials And Methods
The synthesis of difluoromethylselenolated compounds
Difluoromethylselenolated compounds have established worthy consideration for the last few years. Consequently, some research groups individually contributed to the development of new reagents and several synthetic routes have been reported [18].
Difluoromethylselenolation via Se+CF2H
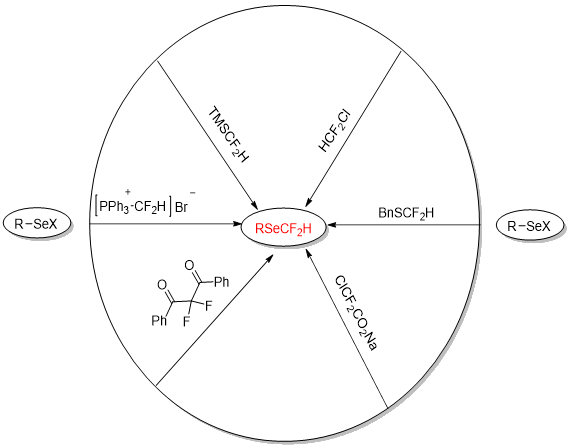
There are two methods for the formation of fluoromethylselenylated molecules: Direct method and indirect method [19]. In the early synthesis of these compounds, it was obligatory first to formulate the selenium-containing substrate and then use different difluoromethylation reagents for the incorporation of CF2H to the selenium atoms of the selenium-containing substrate, which is presented in Figure 2. Simple reaction conditions and cost-effective fluorination reagents distinguish this approach [20].
Figure 2: Indirect approaches of difluoromethylselenolation.
However, due to limited types of selenium-containing substrate, it is challenging to prepare selenylated complex substrates, which dramatically limits the applications and the development of SeCF2H containing molecules in the industrial field.
The deficiency of difluoromethylselenolated reagents and suitable starting materials is considered to be another bottleneck in the synthesis of HCF2Se molecules. In 1985 Suzuki group established a simple method for synthesis of difluoromethyl Selenides from Chlorodifluoromethane (CHClF2) in alkaline solution with a good yield (Figure 3).
Here, CHClF2 was used as a difluoromethylating reagent. The reaction progresses quickly with mild heat evolution and the reaction is very clean with less side-products. Fluoroalkylation of selenolates takes place more readily. This methodology is also useful for aliphatic selenolates. But due to foul odor and high volatility, its scope was further not explored.
Figure 3: Synthesis of difluoromethyl selenides.
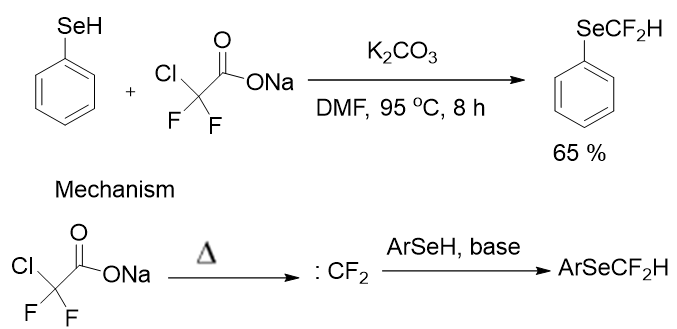
With the disadvantages of the traditional CF2H reagent chlorodifluoromethane gas as an ozone-depleting reagent. Haszeldine was the first to suggest using an alternate reagent like SCDA (Sodium Chlorodifluoroacetate) as a difluorocarbene source. F Greaney group revealed one of the first ways to synthesize SeCF2H-containing compounds from phenylselenol with a yield 65%. In the difluoromethylation of phenylselenol, they used SCDA (Sodium Chlorodifluoroacetate) as a difluorocarbene source; the reaction, as well as its likely mechanism, is depicted in Figure 4. This transformation is both environmentally friendly and cost-effective, and the reaction conditions are straightforward: K2CO3 at 95°C without transition metal catalyst.
Figure 4: Synthesis of SeCF2H containing compound via SCDA.
As for difluoromethylation where several reagents have been presented by some groups, and it was still underexploration. Two effective phosphonium salts have been applied to the difluoromethylation by Xiao group. These reactions occured through the CF2-carbene pathway, that is readily generated from these phosphonium salts. Following that, Hiene research grroup repoted a technique for the difluoromethylation of different thiols and also benzeneselenol by using (difluoromethyl) triphenylphosphonium bromide with mild reaction conditions. The reaction is carried out without the need of a transition metal and with the use of a bench-stable and easily accesible phosphonium salt. Benzeneselenol resulted in a 61% yield of the desired product (Figure 5).
Figure 5: Synthesis of difluoromethyl selenides via phosphonium salt.
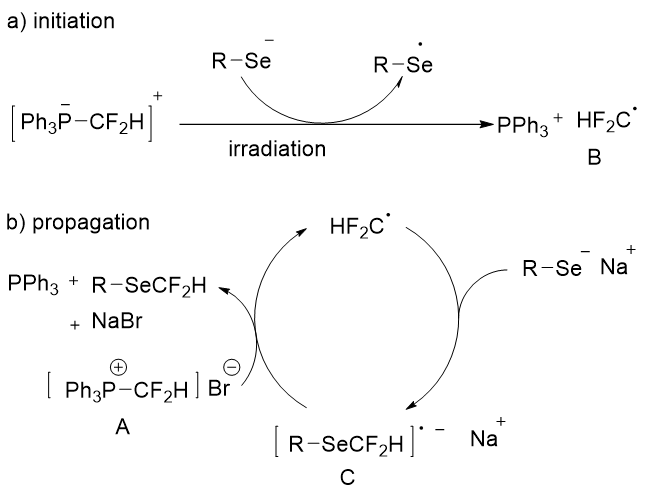
The authors postulated the following mechanism for the difluoromethylation reaction based on the reaction (Figure 6). Irradiation causes electron transfer from the selenolate anion to the phosphonium salt A, resulting in the formation of the difluoromethyl radical B. Direct electron transfer from the selenolate to the phosphonium salt is also useful as an initiation step because the chain is also initiated in the dark, though less efficiently. In a propagation step, radical B reacts with the selenolate anion to create the radical anion C. Single electron transfer from C to the phosphonium salt A affords the corresponding difluoromethylation product along with the radical B, NaBr, and PPh3. It was the first article on an SRN1-type reaction by the use of a phosphonium salt.
Figure 6: Mechanism for the difluoromethylation.
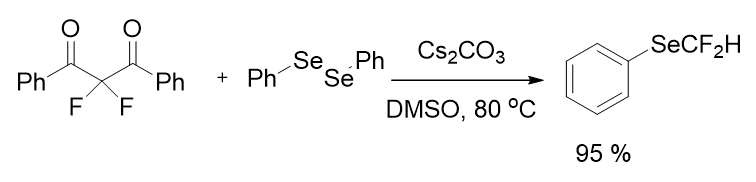
There was still room for more research into transition metal free techniques for nucleophilic CF2H sources. In 2015, Yi gruop also established a method for the synthesis of (difluoromethyl)(phenyl)selane by difluoromethylation of diphenyldiselenide presenting a single example of difluoromethylselenolation. As a nucleophilic difluoromethylation reagent, -Difluorodiaroylmethane was utilized; the reaction was metal-free and temperature dependant. Difluoromethylphenylselane was obtained in a high yield of 95% (Figure 7). This reaction was created primarily to produce (Ar1COCF2SAr) and (Ar1COCF2SAr) (ArSCF2H).
Figure 7: Synthesis of difluoromethylphenylselane.
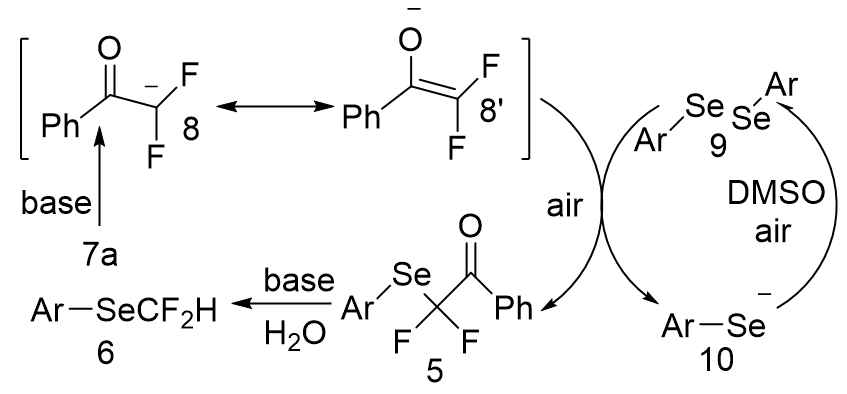
Based on the results, a clear plausible mechanism for this reaction was established (Figure 8). Cs2CO3 first interacts with, -difluorodibenzoylmethane 7a to form, -difluoroenolate intermediate 8, which then reacts with diselenide 9 to produce product 5 and benzeneselenolate 10. The base-induced Haller-Bauer reaction converts the product 5 to difluoromethylselenolated arene 6, while the benzeneselenolate 10 is oxidized to a diselenide 9 and incorporated into the reaction cycle.
Figure 8: Mechanism for the synthesis of 5 and 6 from α, α-difluoro-dibenzoylmethane.
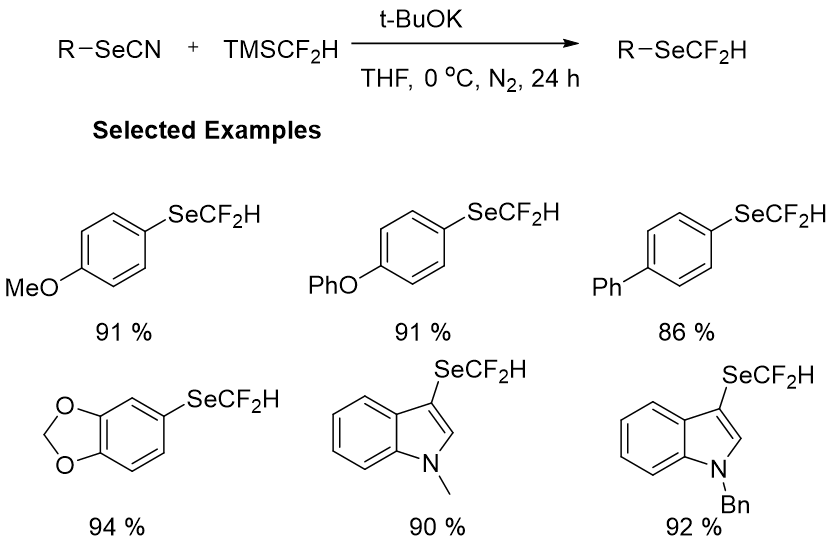
All of the methods presented have limitations in terms of substrate scope, remoteness of starting materials, cost, efficacy, and yielding less HCF2Se-containing products. As a result, developing practical procedures for the production of difluoromethylselenolated molecules was critical. The Zhang group then developed another successful transition metal free technique for the production of aryl and alkyl (RSeCF2H) from selenocyanates (RSeCN) and TMSCF2H. The reaction was carried out in THF at room temperature for 6 hours or at 0 degrees Celsius for 24 hours, yielding a series of difluoromethylselenolated compounds in moderate to excellent yields (Figure 9). The scaled-up formation of 1-benzyl-5-((difluoromethyl)selanyl) indoline and the successful synthesis of difluoromethylselenolated sulfadimethoxine derivatives suggested that this strategy was feasible. The advantages of this reaction include mild reaction conditions, a wide range of substrates, strong functional group tolerance, and high efficiency. This procedure provided a number of novel difluoromethyl selenoethers, allowing for faster use of these molecules in life science (Figure 10).
Figure 9: Synthesis of difluoromethyl selenoethers from different types of aryl and alkyl selenocyanates.
Figure 10: Synthesis for the production of aryl and alkyl from selenocyanates.
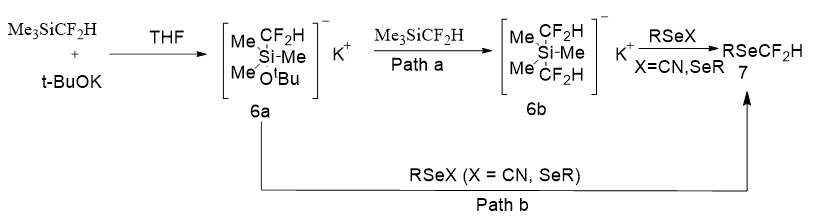
On the basis of the above reaction and the preceding reports, a plausible mechanism was proposed for the difluoromethylation (Figure 11). Initially, t-BuOK coordinates with the siliconcenter of Me3SiCF2H to get a pentacoordinate silicate (10a). Reaction of 10a with another equivalent of Me3SiCF2H generates an intermediate K[Me3Si(CF2H)2] (10b), which undertakes a nucleophilic attack at the selenium atom of RSeX (X¼CN, SeR) to give the desired product RSeCF2H. However, direct formation of RSeCF2H by the difluoromethylation of RSeX with 10a cannot be omitted in the reactions at this stage. Moreover, minor RSeSeR species were detected in some reactions might be generated in situ from RSeCN, which could be further transformed to RSeCF2H under the reaction conditions.
Figure 11: Mechanism for the synthesis of RSeCF2H from R-SeX and Me3SiCF2H/t-BuOK.
Difluoromethylselenolation via in situ generated HCF2SeCl
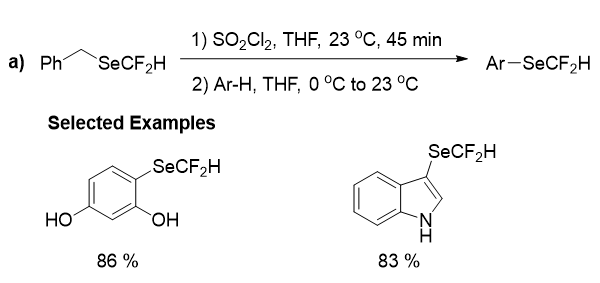
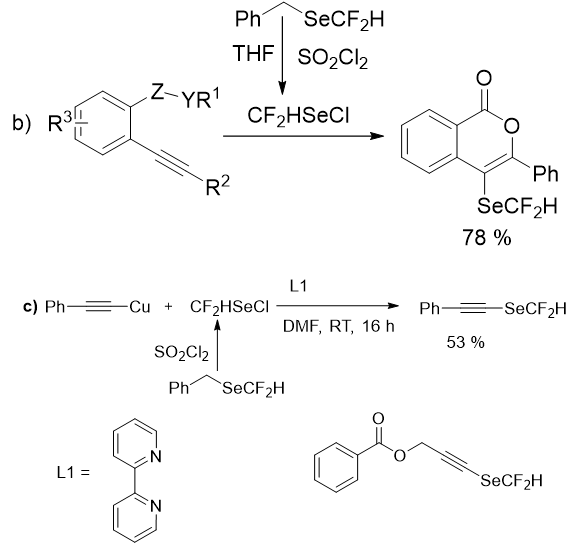
This underdevelopment approaches to synthesize difluoromethylselenolated compounds in the absence of transition metals, were partially because of the absence of effective reagents to perform difluoromethylselenolation reactions. The remarkable success here was the in situ generation of the volatile electrophilic reagent (HCF2SeCl). So, in 2016 Billiard group developed an approach for the synthesis of CF3SeCl which was based on the oxidative chlorination of benzyltrifluoromethyl selenide (this compound is simple and can be synthesis from CF3SiMe3 and benzylselenocyanate). And they established a one-pot method based on this starting material by evading the separation of CF3SeCl, to achieve trifluoromethylselenolation of aromatic compounds. In addition, this methodolgy was prolonged to other fluoroalkylselenolations. Thus benzylbromodifluoromethyl selenide were used as starting material to achieve bromodifluoromethaneselenylated molecules as targeted products in moderate to high yields. Similarly, benzyldifluoromethyl selenide was used for the access of CF2HSeCl, which was further used for the difluoromethylselenolation of few aromatic compounds with a limited substrate scope (Figure 10). Again Billiard and his group disclosed a transition metal-free difluoromethylselenolation of alkyne with CF2HSeCl reagent to form an isocoumarin derivative and an oxidant-free difluoromethylselenolation of alkynyl copper(I) complex to yield a difluoromethyl alkynyl selenide (Figure 12). After that, Tlili group reported the successful one-pot procedure based on the in situ generation of CF2HSeCl for the perfluoroalkylselenolation of alkynyl copper (I) compounds (Figure 12).
The challenging SeCF2H alkyne product was also obtained in good yield but only two examples of SeCF2H limited the reaction scope.
Results And Discussion
Difluoromethylselenolation via R-H+[Se]+[CF2]
For the time being there were two key routes for the difluoromethylselenolation. The most common route was difluoromethylation of phenylselenol and a series of difluoromethylating reagents such as HCF2Cl, ClCF2CO2Na, TMSCF2H and PhP3CF2HBr had been developed for this purpose.
Figure 12: Difluoromethylselenolation via in situ generated HCF2SeCl.
Another approach was the in situ generation of CF2HSeCl with difluoromethylselenolation reagents, for example benzyl(difluoromethyl)selane. Nevertheless, these techniques need synthesis of volatile CF2HSeCl. Temporarily, pre-installation of difluoromethylselenolating reagents was not easy. It became necessary to develop difluoromethylselenolating group precisely from readily accessible starting materials.
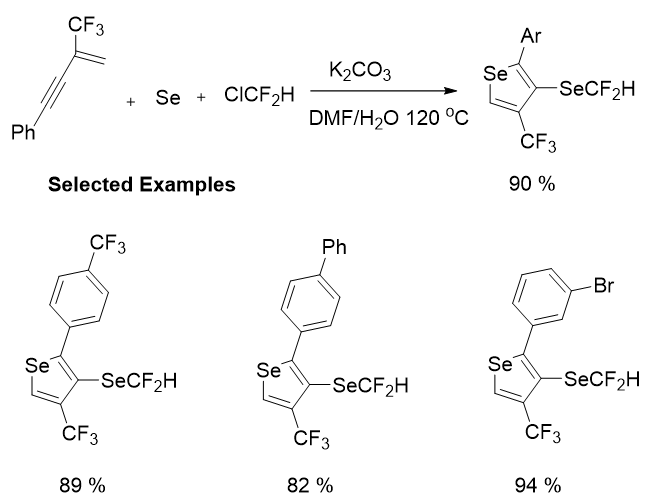
Then, a contradictory method for the precise formation of SeCF2H groups on heteroaromatics caused in situ from CF3-containing 1,3-enynes was established by Song’s group recently. Reactions of easily accessible 1,3-enynes with Se and ClCF2H (as a cheap difluoromethylation reagent) gave a series of 3-SeCF2H-4-CF3-selenophenes by same approach under the same standard conditions with moderate to high yields. Irrespective of whether the substituents on the aromatic rings were electron withdrawing, electron donating or neutral, the resultant substrates can ensue easily to render the products in good to high yields. Specially, halogens (Br and Cl) can be retained well in the optimized conditions, which afford practicability for additional structural elaborations (Figure 13).
Figure 13: Regioselective synthesis of 3-SeCF2H-4-CF3-selenophenes.
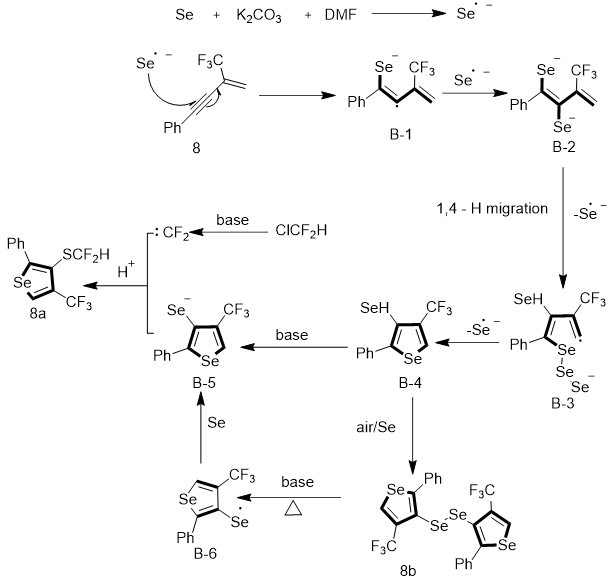
Several control experiments were conducted to gain perceptions of the reaction mechanism. On the basis of the reported literature and our observation, a plausible reaction mechanism is proposed in Figure 14. First, Se•− in situ generated from Se in basic conditions reacts with 1,3-enyne 8 to produce the radical anion intermediate A-1. Another Se•− attacks intermediate B-1 to get intermediate B-2, which transforms to intermediate B-3 via Se−Se bond homolysis and 1,4-hydrogen migration. The intramolecular radical coupling of B-3 provides intermediate B-4 through Se−Se bond homolysis and releasing Se•−. Then, oxidation of intermediate B-4 occurs by air or Se to synthesize the diselenides. Meanwhile, selenium anion B-5 is confined by the difluorocarbene generated in situ, which was formed from B-4 in basic conditions and ultimately resulting in difluoromethylselenolated compound 8a. On the other hand, 8b undergoes a homolytic process to reduce free radical B-6 to B-5 by Se.
This conversion is a free radical process, and an excess of Se acts as a reducing agent to make this SN2 reaction very efficient. Moreover, experiments suggested that the Se comes from the starting material, not from the external Se source.
Figure 14: Mechanism for the synthesis 3-SeCF2H-4-CF3-selenophenes.
Difluoromethylselenolation via R-H+SeCF2H reagent
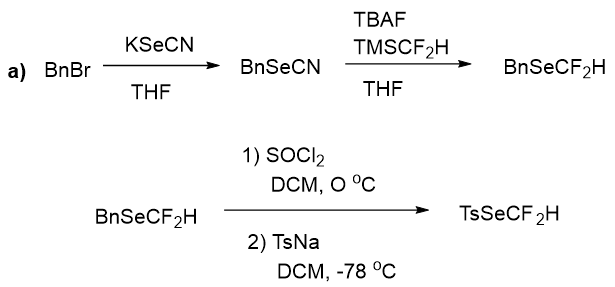
As methologies for insertion of the SeCF2H group have gained great considerations in the synthetic society. Though, direct incorporation of SeCF2H group by means of a shelf-stable reagent was not reported before. The direct incorporation of SeCF2H is the most sophisticated approach. Therefore, some research groups individually contributed in establishing new reagents and methodologies for this purpose. Herein, this method aims to avoid the usage of starting materials, which involves additional synthetic steps. Recently, Zhao group35 developed a new approach of visible-light photocatalytic difluoromethylselenolation of arylamines through in situ generation of aryldiazonium salts by using Se-(difluoromethyl) 4-methylbenzenesulfonoselenoate, this reagent was also prepared for the first time. The Preparation of the SeCF2H reagent was accomplished by the use of KSeCN, benzyl bromide (BnBr), CF2HSiMe3, and sodium 4-toluenesulfinate (TsNa), employing a practice likewise for the preparation of Se-(trifluoromethyl) 4-methylbenzenesulfonoselenoate as highlighted in Figure 15.
Figure 15: Preparation of reagent Difluoromethylselenolation.
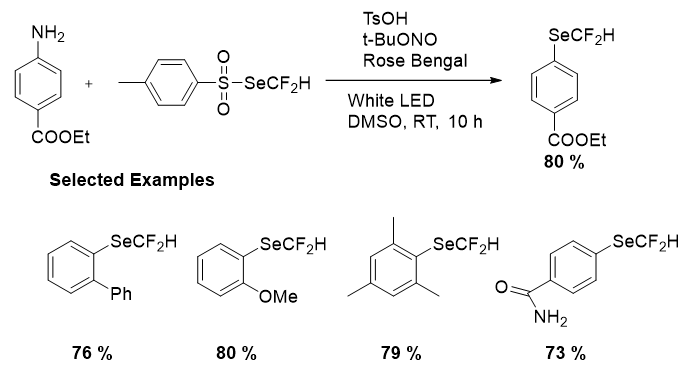
Once the SeCF2H reagent was prepared, the difluoromethylselenolation of ethyl 4-aminobenzoate with 4-methylbenzenesulfonic acid (TsOH), Tert-Butyl Nitrite (TBN) was attained, in the existence of Rose Bengal (RB) under the white LED in Dimethyl Sulfoxide (DMSO) at room temperature. All the reagents used were inexpensive, readily available and shelf-stable. After the optimization the generality of the reaction was scrutinized by employing a number of arylamines as shown in Figure 16. Anilines having electron-donating and electron-withdrawing groups, including meta-, ortho- and para-substituted anilines, were suitable for the reaction conditions and gave the consistent difluoromethylselenolated products in modest to excellent yields. Remarkably, sterically hindered anilines were efficiently converted into the relevant products in good to excellent yields.
Figure 16: Difluoromethyl selenolation of arylamines and substrate scope.
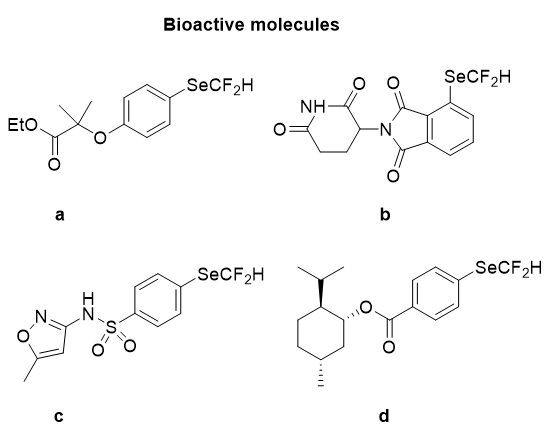
Next, the developed method of Zhao group was further used for the synthesis of pharmaceutically significant molecules, the functionalization of well-known bioactive scaffolds was carried out and successful insertion of the SeCF2H group into a clofibrate derivative (a), pomalidomide (b), sulfamethoxazole (c), and L-menthol derivative (d) in moderate to good yields was achieved as presented in Figure 17.
Figure 17: Difluoromethyl selenolation of bioactive molecules.
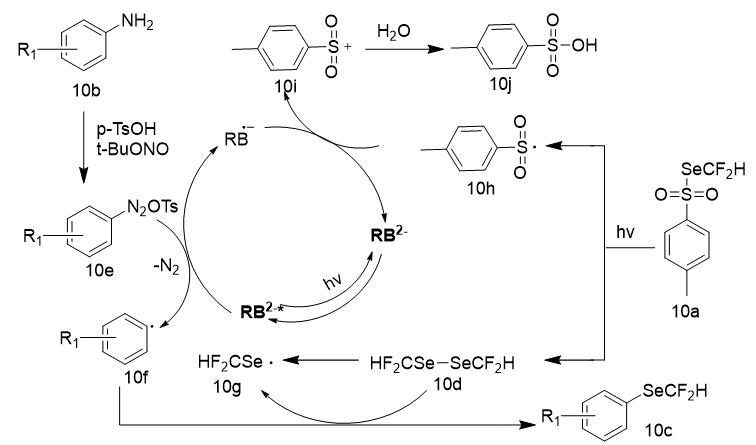
The authors also investigated the mechanism of the reaction, a series of experiments were carried out for this purpose. Based on the previous mechanisms in the literature and aforementioned results, a plausible mechanism for the selenodifluoromethylation reaction was proposed (Figure 18). The arylamine 10b reacts with TsOH and TBN to form an arenediazonium salt 10e. The photocatalyst Rose Bengal (RB) undergoes excitation under visible-light irradiation to RB*, which through a Single Electron Transfer (SET) with arenediazonium salt 10e leads to the generation of RB•− and aryl radical 10f, which reacts with 1,2 bis(difluoromethyl)diselane 10d generated by the homolysis of the reagent 10a under visible light to deliver the corresponding aryl difluoromethylselenol ether 10c and difluoromethylselenol radical 10g, which can self-combine to form 10d. Subsequently, the sulfone radical 10h undergoes a Single Electron Transfer (SET) with the radical anion of the photocatalyst (RB•−), which leads to the formation of sulfite cation 10i along with the regeneration of the photocatalyst. The reaction of the sulphite cation 10i with water affords TsOH (10j).
Figure 18: Plausible mechanism of Rose Bengal-catalyzed Selenodifluoromethylation.
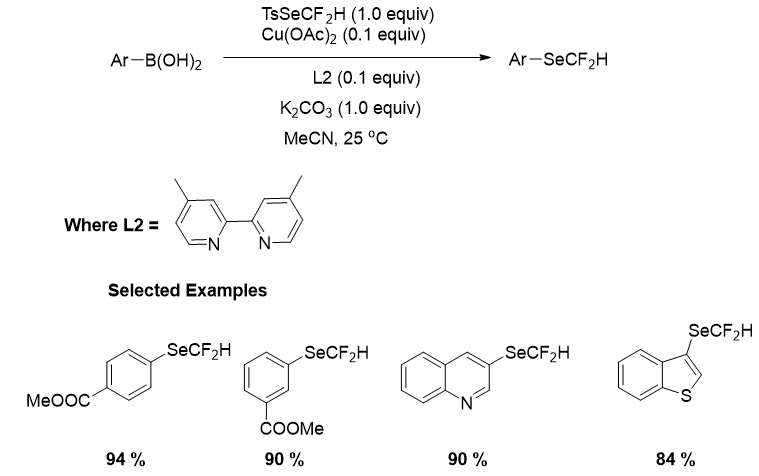
In 2021, the Zhao group once again used TsSeCF2H as a difluoromethylselenolation reagent to achieve the first copper-catalyzed direct difluoromethylselenolation of aryl boronic acids. This reaction is an alternate and realistic technique for the synthesis of aryl difluoromethylselenylether due to the inexpensive and widely available chemicals, broad substrate scope, and mild reaction conditions. In this reaction, phenyl boronic acids with electron donating or electron withdrawing substituents in the para-, meta-, or ortho positions were well tolerated, yielding high yields of the desired products (Figure 19).
Figure 19: Copper-catalyzed direct difluoromethylselenolation of aryl boronic acids.
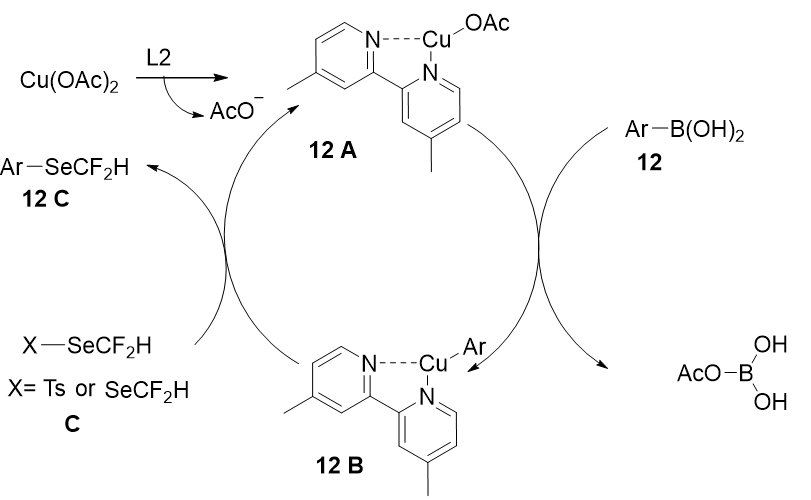
The authors performed several controlled experiments to completely understand the reaction and based on previous literature and the performed experimental results, a plausible reaction mechanism for this transformation was proposed (Figure 20). The active catalytic species, 16-electron copper complex A, was first produced in situ. The intermediate 12B was formed during transmetallation between compound 12A and the aryl boronic acid. Finally, 12B interacted with 1,2-bis(difluoromethyl)diselane or trifluoromethylselenolated reagent C to generate the desired product 12C.
Figure 20: Plausible mechanism difluoromethylselenolation of aryl boronic acids.
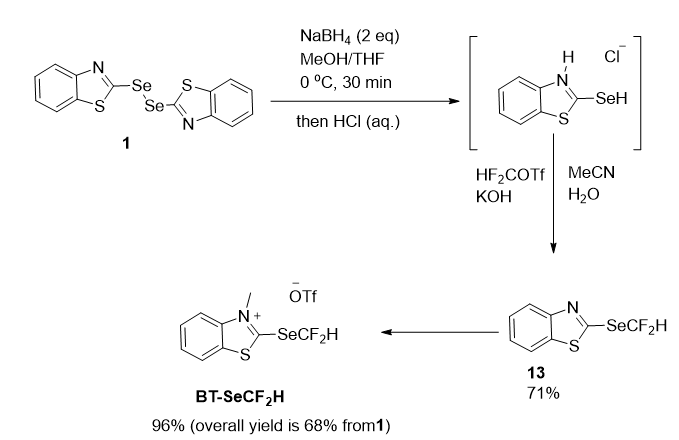
The benzothiazolium salt BT-SeCF2H was introduced by Matthew group in 2021 as a competent nucleophilic reagent for transferring difluoromethylselenyl groups into organic compounds. Deoxygenative substitution of widely available carboxylic acids yielded SeCF2H-containing selenoesters, whereas silver catalysis allowed the efficient production of (difluoromethyl)selenoethers, which contained the well-known electrophilic reagent BnSeCF2H, straight from simple alcohols. These deoxygenative reactions are the first nucleophilic difluoromethylselenylation pathways to be reported, opening up new avenues for the production of important fluorinated chemicals.
Firstly, a two-stage strategy was planned, with a neutral non-methylated benzothiazole intermediate. Using NaBH4, bis(benzothiazole)diselenide was reduced to selenol in the first step. Following precipitation as the benzothiazolium chloride adduct, treatment with difluorocarbene derived from HCF2OTf under basic conditions yielded the stable heteroarene 13, which was extracted in 71% yield using column chromatography. BT-SeCF2H was obtained in 96% yield after N-methylation with methyl trifluoromethanesulfonate in CH2Cl2 at room temperature and precipitation with diethyl ether (overall yield of 68% from 1, 35 mmol scale) (Figure 21). When held under air at room temperature for several months, BT-SeCF2H was produced as an off-white solid that required no additional purification.
Figure 21: Synthesis of BT-SeCF2H.
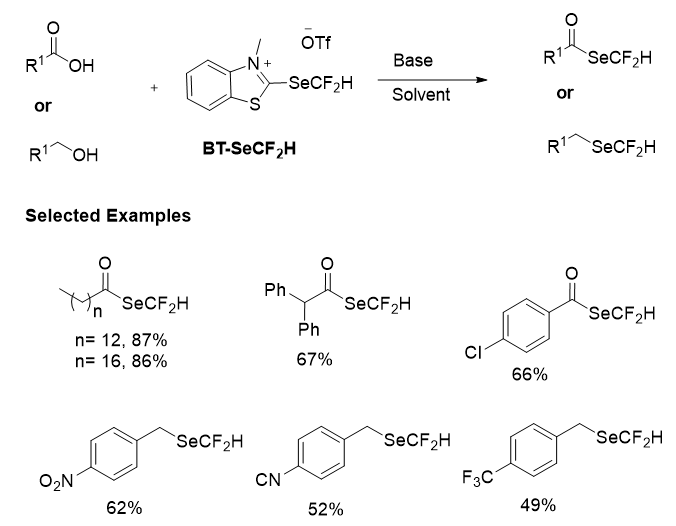
Once BT-SeCF2H was synthesized, then its reactivity as a nucleophilic difluoromethylselenylating reagent was investigated. The scope of the deoxygenative difluoromethylselenylation reaction was then tested with a range of carboxylic acid and alcohol derivatives (Figure 22). A wide selection of aliphatic substrates could be successfully converted into the corresponding (difluoromethyl)selenoesters in excellent yields. Primary, secondary and even tertiary derivatives were all tolerated providing selenoesters in excellent yield after column chromatography.
Figure 22: Deoxygenative difluoromethylselenylation of carboxylic acid and alcohol derivatives.
Approach towards Monofluoromethylselenylation
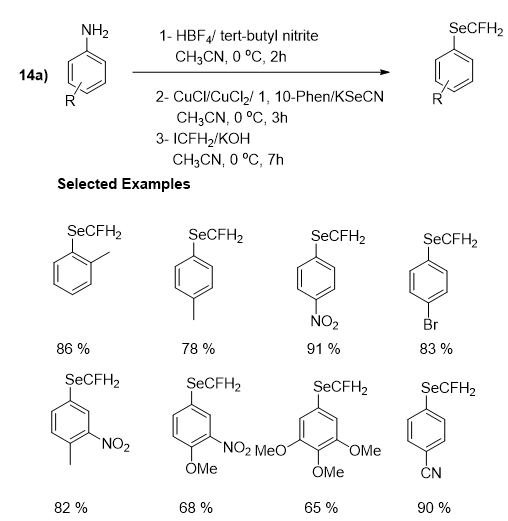
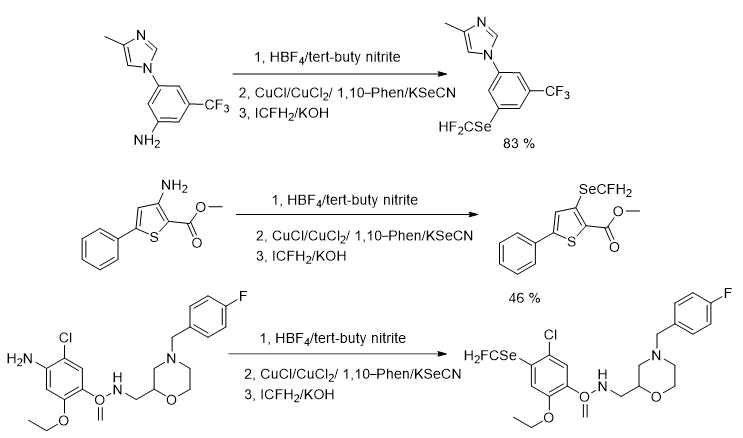
A number of research articles have been reported the methodologies of the introduction of fluoroalkylseleno groups into small molecules, comprising Trifluoromethylseleno (-SeCF3) and Difluoromethylseleno (-SeCF2H) groups. Organoselenocyanate has been reported as a selenium reagent in these approaches. These methods have numerous advantages, comprising mild reaction conditions, broad substrates scope, excellent functional group tolerance, and extraordinary proficiency. Similarly, the introduction of SeCFH2 group into organic molecules have significant applications in the field of medicinal as well as agro chemistry. But, unlike SCFH2, SeCFH2 group has been less studied. Though, the insertion of the (SeCFH2) group into small molecules is challenging and only a single article has been published so far. In 2019, Yi research group presented the incorporation of the monofluoromethylseleno group (-SeCFH2) to aryl and alkyl halides through one-pot multistep synthesis using organoselenocyanate and ICF2H, this methodology proved to be very useful and feasible as excellent product yields and a wide range of functional group compatibility were achieved. Furthermore, the fruitful synthesis of SeCFH2 containing drug-like compounds enhanced the value of this approach. Additionally, it gave a number of novel monofluoromethyl selenoethers, which would fast-track the use of these compounds in the field of life science. In the first section they described three step synthesis of monofluoromethyl(o-tolyl) selane, by following the methods introduced by Yi and Rueping’s group in the primary two steps respectively, and by adding ICFH2 in the third step of the reaction for the introduction of -CFH2 group with the corresponding selenocyanates. After optimization of the reaction, different substrates of aniline having various functional groups were examined. In general, a range of aryl substituted amines were appropriate and accomplished the desired products in good to excellent yields. The reaction was suitable for all substrates bearing different electron-donating or electron-withdrawing groups, a variety of well-known functional groups such as methyl, methoxy, halogen, cyano and nitro were all well-tolerated. Likewise, disubstituted and trisubstituted aniline could also be transformed to the consistent products in good yields (Figure 23).
Figure 23: Monofluoromethy lselenolation of anilines.
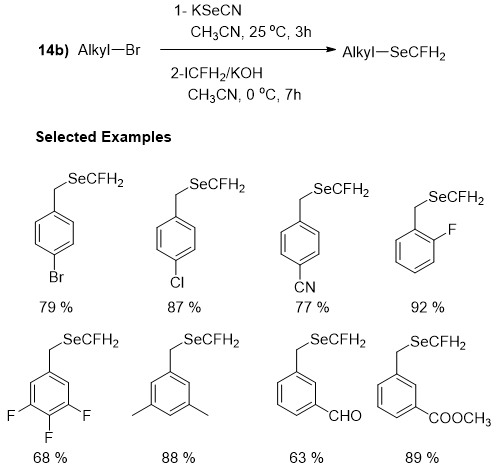
While, in the second section monofluoromethylselenolation of alkyl bromides was accomplished via one-pot two steps with the standard reaction. Similarly, the reaction offered excellent product yields for both electron-donating or electron-withdrawing groups. Disubstituted benzyl and trisubstituted benzyl bromide could also be transformed to the corresponding products in moderate yields (Figure 24).
Figure 24: Monofluoromethylselenolation of alkyl bromides.
To further determine the efficacy of the reaction in a late-stage modification, few medicinal compounds were explored. Nilotinib intermediate (use as an anticancer drug) and metsulfuronmethyl intermediate (as pesticides intermediate) were also suitable for this reaction and delivered the resultant products in 83% and 46% yields, correspondingly. Moreover, complex molecule such as mosapride (a gastroprokinetic agent), was selectively transformed at the amino group to achieve the monofluoromethylselenolated product in 78% yield. Vitamin E derivative also gave monofluoromethylselenolated product in excellent yield. These reactions deliver feasible approach to functionalized molecules by a late-stage monofluoromethylselenolation reaction (Figure 25).
Figure 25: Monofluoromethyl selenolation of drug-like compounds.
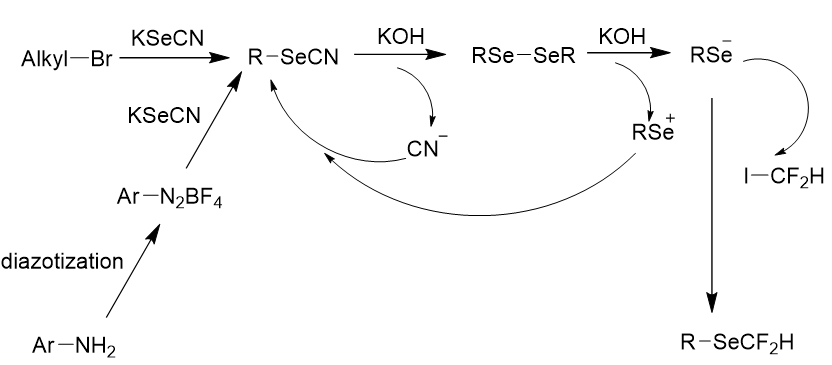
The authors conducted a series of experiments to completely understand the reaction, specially the establishment of monofluoromethyl selenoethers from RSeCN and ICFH2, the reaction was examined in the occurance of RSeCN, ICFH2 and KOH. RSeSeR was generated at the initiation of the reaction. As the reaction progressed, RSeCFH2 was developed with a reduction in RSeSeR. It specified that RSeCFH2 was transferred from RSeSeR. A few control experiments were accomplished to gain more insights. Reaction of selenocyanatobenzene with KOH could present PhSeSePh in 92% yield. Reaction of PhSeSePh and ICFH2 was performed in the absence of KOH to confirm that KOH indeed promoted the monofluoromethylation. Only traces of desired product were found, while 90% yield was observed with 10equivalent of KOH. On the basis of these observations, a plausible mechanism for monofluoromethylselenolation was proposed (Figure 26). Primarily, RSeSeR was formed from RseCN and KOH. KOH promoted the nucleophilic attack of RSe- to ICFH2 to form RSeCFH2 products. Meanwhile, CN- in the reaction mixture reacted with RSe+ to generate RSeCN.
Figure 26: Plausible mechanism for Monofluoromethyl selenoethers of aryl and alkyl.
Approach towards Difluoromethyl Tellurides
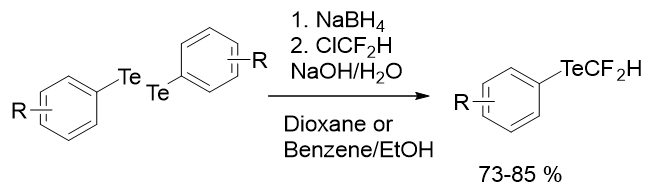
Tellurium is an infrequent element which has regarded as an unnecessary, toxic element and has received less considertion than that of selenium. Several literature reports also stressed that its compounds are highly toxic. Tellurium composes both inorganic and organic derivatives. The biological effects of tellurium and few of its organic derivatives have been studied which have proven some interesting and promising applications. As an example, it can be highlighted the uses of organotellurides and diorganotellurides as an anti-oxidant and also as protease inhibitors and its application in disease models. Similar to selenim, fluorotelluranes are also less explored as compared to fluorothiols. Only a few examples of TeCF2H compounds are up to date. As mentioned above difluoromethyl tellurides still need to explore as only single example for the formation of CF2H tellurium compounds was presented more than 30 years ago by Suzuki group (Figure 27). They established an easy approach for the synthesis of difluoromethyl Tellurides from chlorodifluoromethane (CHClF2) in alkaline solution with a good yield. The reaction progresses quickly with mild heat evalution and the reaction is very clean with minor side-products. Fluoroalkylation of tellurolates takes place more readily than selenolates. This methodology is also useful for aliphatic selenolates and Tellurolates. But due to stinky smell and high volatily its scope was further not explored.
Figure 27: Synthesis of Difluoromethyl Tellurides.
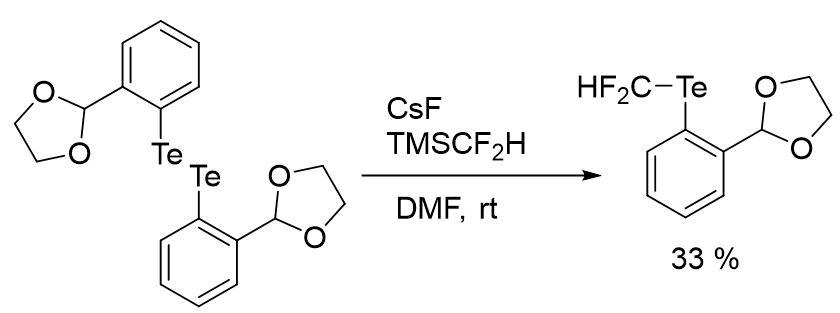
As the freon gas required high pressure, so keeping in view the importance and the applications of difluoromethyl tellurides Togni group presented the formation of tellurium derivatives having fluorinated groups, same as the hypervalent iodine congeners. Although tellurium compound directly involving a CF2H group attached to tellurium atom was known, but there were no reports on hypervalent CF2H tellurium compound. Togni group obtained CF2H aryltellurium(II) species bearing various functional groups interacting with the Te atom. The incorporation of the fluorinated group depends on the use of the consistent dimethylsilyl precursors. Therefore, the conditions established for difluoromethylation of disulfides by Zhang group, were used for to synthesize the CF2H tellurium compound in moderate yield of 33% (Figure 28).
Figure 28: Synthesis of Hypervalent CF2H Tellurium compound.
Conclusion
As highlighted in this review, the insertion of the HCF2Se, HCF2Te and H2CFSe groups into organic compounds can significantly alter their biological and physicochemical behaviours. The development of HCF2Se containing drugs and agrochemicals have encouraged this topic into the advancement of effectual methodologies for direct difluoromethylselenolation reactions. However, noteworthy achievements have been made in the last few years, additional progresses are still required. The article will help in the development of new fluorinated compounds with unique properties and new functions. It is our anticipation that this review will inspire chemists to develop novel and exciting methodologies for direct incorportion of SeCF2H, TeCF2H and SeCFH2 groups.
Conflect Of Interest
The authors declare no competing financial interest.
Acknowledgement
Both listed authors have contributed substantially to the design, performance, analysis, or reporting of the work. We gratefully acknowledge the National Natural Science Foundation of China (21776138, 22078161, 22108124), the Fundamental Research Funds for the Central Universities (30920021124, 30918011314), the Natural Science Foundation of Jiangsu (BK20180476), the Postdoctoral Science Foundation Funded Project (2019M661848), the Priority Academic Program Development of Jiangsu Higher Education Institutions, and the Centre for Advanced Materials and Technology in Nanjing University of Science and Technology for their financial support.
References
- Erickson JA, et al. J Org Chem. 1995;60(6):1626-1631.
- Li J, et al. Chem Sci. 2018;9(26):5781-5786.
[Crossref] [Google Scholar] [PubMed]
- Liu F, et al. Org Lett. 2018;20(19):6270-6273.
[Crossref] [Google Scholar] [PubMed]
- Okui S, et al. Can J Chem. 2008;7:371.
- Morita K, et al. Agric Biol Chem. 1987;51(5):1339-1343.
- Yagupolskii LM, et al. J Fluor Chem. 2001;109(1):87-94.
- Huang Z, et al. Org Lett. 2017;19(4):934-937.
[Crossref] [Google Scholar] [PubMed]
- Ashton MJ, et al. J Med Chem. 1996;39(25):4888-4896.
[Crossref] [Google Scholar] [PubMed]
- Howland WC. Clin Exp Allergy. 1996;26:18-22.
[Crossref] [Google Scholar] [PubMed]
- Zhou J, et al. Org Process Res Dev. 2014;18(8):928-933.
- Jung M, et al. Pharm Pharmacol Commun. 2000;6(5):217-221.
- Robichaud J, et al. Bioorg Med Chem Lett. 2007;17(11):3146-3151.
[Crossref] [Google Scholar] [PubMed]
- Hernandez-Ayala LF, et al. J Mol Struct. 2020;1205:127449-127478.
- Schacht J, et al. Adv Integr Anat Evol Biol. 2012;295(11):1837-1850.
[Crossref] [Google Scholar] [PubMed]
- Singh N, et al. Neuropsychopharmacology. 2016;41(7):1768-1778.
[Crossref] [Google Scholar] [PubMed]
- Angeli A, et al. Bioorg Med Chem. 2017;25(8):2518-2523.
[Crossref] [Google Scholar] [PubMed]
- Lynch ED, et al. Hear Res. 2005;201;81-89.
[Crossref] [Google Scholar] [PubMed]
- Singh N, et al. Nat Commun. 2013;4(1):1-7.
[Crossref] [Google Scholar] [PubMed]
- Hansch C, et al. Chem Rev. 1991;91(2):165-195.
- Holben DH, et al. J Am Dietet Assoc. 1999;99:836-843.
[Crossref] [Google Scholar] [PubMed]