Original Articles: 2025 Vol: 17 Issue: 2
Progress in Synthesis of Natural Algicide Bacillamides and New Green Technology for Biomimetic Preparation
Shuo Gao, Yue Jiang, Virinder S. Parmar, Yu Wang, Hui-jing Ren, Pei Cao*
Department of Pharmacy, Guangxi University of Chinese Medicine, Nannin, China
- Corresponding Author:
- Pei Cao
Department of Pharmacy,
Guangxi University of Chinese Medicine, Nannin,
Nannin,
China
Received: 19-Apr-2024, Manuscript No. JOCPR-24-132612; Editor assigned: 22-Apr-2024, PreQC No. JOCPR-24-132612 (PQ); Reviewed: 06-May-2024, QC No. JOCPR-24-132612; Revised: 08-Jan-2025, Manuscript No. JOCPR-24-132612 (R); Published: 15-Jan-2025, DOI:10.37532/0975-7384.2025.17(2).232.
Copyright: © 2025 Gao S, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The explosive growth of algae resulted from the eutrophication of water bodies occurs frequently around the coastal area in recent years, which severely damages the balance of ecosystems. The classical methods exhibit various restrictions during the prevention procedures under different algae density conditions in the water body at different periods, such as the remaining residues for chemical reagents, the high cost of manpower or big volume of equipment for physical patterns and the instability along with slow effectiveness for biological strategies. The application of new, efficient and safe algal bloom prevention under controlling is a hot and challenging topic currently. The extracellular protease and the secondary metabolite of marine bacteria were thus effectively investigated for the leading compounds with algicidal activity. Considering their low toxicity, high selectivity and environment-friendly properties, the use of natural bacillamides and their analogues as algicides has proven to be particularly attracting. The relating synthesis were carried out for the much lower contents from diversified natural resources. After summarizing the progress in synthesis of natural algicide bacillamides from 2005 to 2023, a new green technology was realized for biomimetic preparation of the key intermediate of thiazole units. The mechanism was discussed for this novel methodology with the potential for the industrial production in large scales.
Keywords
Algicide; Bacillamides; Synthesis progress; Green technology; Biomimetic preparation
INTRODUCTION
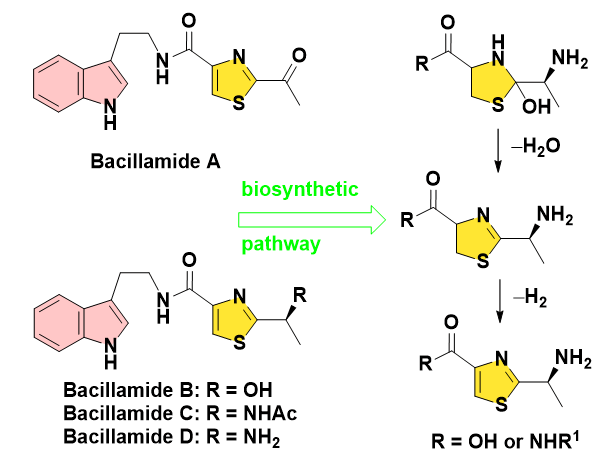
The Harmful Algal Blooms (HABs) in coastal areas exert their influence on both ecosystems and human health. Recently, HAB formation has been continuously enhanced in frequency, intensity and geographical distribution. Anthropogenic factors including discharge from wastewater treatment, shipping or aquaculture, play important roles in the promotion and development of HAB events. Desalination plants or coastal farms are also affected markedly. Thus, the corresponding surveillances and managing practices are usually much in demand. The development of new, efficient, safe HAB preventions and control protocols as a hot topic still remains challenging. Generally, conventional methods have various disadvantages such as chemical residues for chemical strategies, the high cost of manpower or equipment for physical modes, together with the instability and slow effectiveness for biological patterns. Consequently, novel technologies are highly desired and paid special attentions [1-3]. Many marine bacteria act as algaecides, which can effectively control algal reproduction in the natural environment. Therefore, their extracellular protease and the secondary metabolite were extensively investigated for the leading candidates with inhibitory activity for HABs [4]. In 2003, Bacillamide A was isolated from a marine Bacillus endophyticus displaying antibiotic activity against dinoflagellates and raphidophyte [5]. In 2007, Socha obtained its homologues Bacillamide B and C from the ultra-high salinity microbes [6]. These alkaloids prominently feature a dipeptide skeleton incorporating a central thiazole motif and a C-terminus tryptamide. The biosynthetic pathway reveals the generation of a hydroxylated thiazolidine intermediate, which is subsequently dehydrated and dehydrogenated to provide the final thiazole-containing product like Bacillamide D (Figure 1) [7,8]. In consideration of the limited availability of family members from natural source as a focus in this field, the relational bioactivity studies along with the further applications for bacillamides were usually restricted. While summarizing the progress in synthesis of natural algicide bacillamides from 2005 to 2023, a new green technology was developed by our team for biomimetic preparation of the key intermediate of thiazole units. The mechanism was proposed for this novel methodology with the potential for the industrial utilization in large scales.
Figure 1: Bacillamide alkaloids and the relational biosynthesis pathway.
As to the preparation of bacillamides, many researchers designed different schemes. However, most of the reaction routes are complicated, the conditions are relatively harsh, some use special and expensive transition metal catalytic reagents, the overall yield is usually low, the practical value is not attractive. Presently, the traditional synthetic procedures or the conventional fermentation and bioengineering strategies are tedious to meet further pharmacological and toxicological needs. There is still a gap between achieving scientific research and industrial-grade production goals with practical values, especially for industrialized large-scale applications by comprehensive assessment.
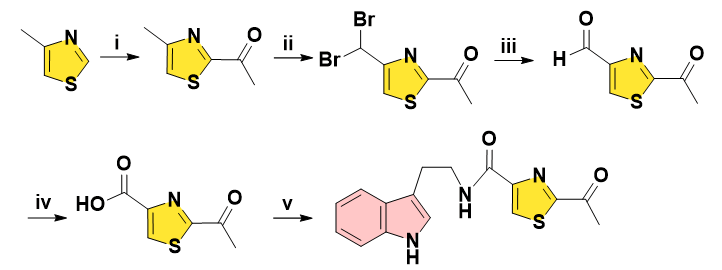
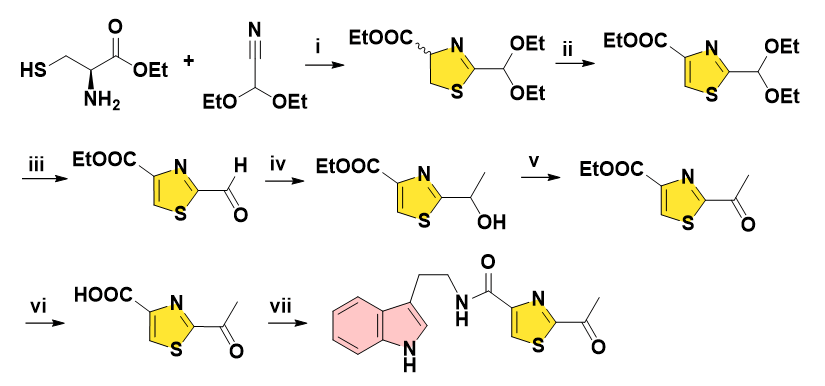
In 2005, Lobo and co-workers reported the synthesis of Bacillamide A in 5 steps, expensive 4-methylthiazole as starting material underwent lithiation followed by treatment with EtOAc to get the C-acetylated thiazole. N-bromosuccinimide secured dibromide with moderate yield, which was converted into aldehyde by solvolysis in the presence of AgOAc. Subsequent oxidation in H2O2 and NaClO2 solution afforded carboxylic acid being coupled with tryptamine finally (Figure 2) [9].
Figure 2: Reagents and conditions: (i) BuLi, dry diethyl ether, ethyl acetate, -78ºC, 64%. (ii) NBS, AIBN, CCl4, reflux, 54%. (iii) silver acetate, acetone-water, 82%. (iv) 50% H2O2 solution, buffer solution of NaH2PO4 (pH 4.3), NaClO2, 0ºC to rt, 95%. (v) Pivaloyl chloride, DIEA, tryptamine, DCM, 0ºC to rt, 60%.
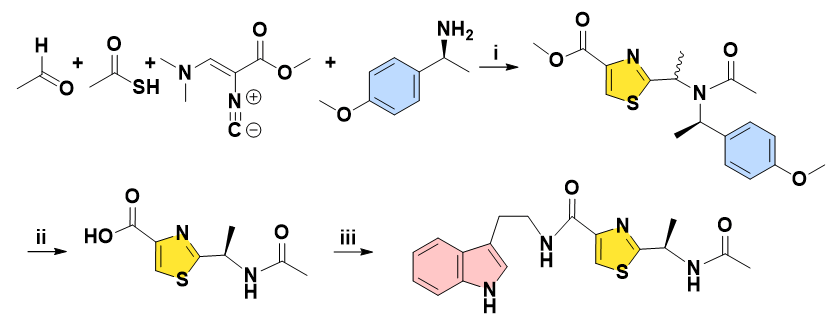
In 2010, Dömling and co-workers designed the chiral resolution for derivatives of Bacillamide C by 3 steps in total, which relies on one key transformation constituting a thiazole Ugi multicomponent reaction. Special isocyanide reagents were generally toxic and irritant, moreover, with comparably higher costs. The efficiency for ultimate amidation is slightly lower (Figure 3) [10].
Figure 3: Reagents and conditions: (i) MeOH, r.t. 60%. (ii) TFA, r.t. (iiia) LiOH, THF/H2O, 58% over 2 steps. (iiib) CDI, HOBT, DIPEA, tryptamine, 30% yield with 94% ee.
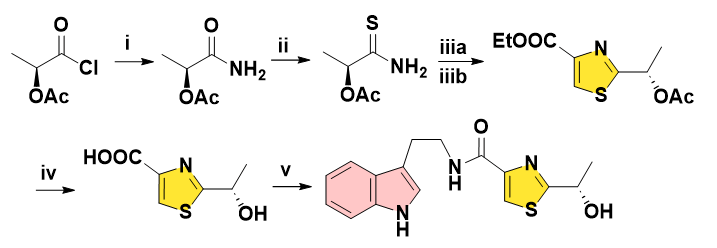
In 2010, Bray and co-workers developed the 5-step route to get Bacillamide B, the unstable, irritating, toxic acetyl chloride was adopted for initial amidation, followed by a Hantzsch reaction using excess Lawesson’s reagent and bromopyruvate under harsher acidic condition. Finally, the dipyridyl disulfide and Ph3P-mediated coupling furnished the amide with moderate yield (Figure 4) [11].
Figure 4: Reagents and conditions: (i) NH3, DCM, 20°C, 1 h, 88%. (ii) Lawesson’s reagent (2 eq), 1,4-dioxane, 20°C, 16 h, 69%. (iiia) ethyl bromopyruvate, ethyloxirane, i-PrOH, 60°C, 30 min. (iiib) TFAA, 20°C, 30 min, 58% over 2 steps. (iv) 2 M LiOH, THF–MeOH, 20°C, 24 h, 80%. (v) dipyridyl disulfide, Ph3P, tryptamine, DCM, 20°C, 16 h, 54%.
In 2012, Fache and co-workers employed a 7-step process to give Bacillamide A. Cysteine and 2,2-diethoxyacetonitrile were catalyzed by phosphotungstic acid (2%) under microwave irradiation to obtain the 2-arylthiazoline, whose dehydrogenation was achieved by equivalent amount of BrCCl3. The branching acetyl group was introduced through multi-steps with totally inefficient transformations, including the methylation by unstable Grignard reagent and the oxidation by dangerous Dess-Martin reagent (Figure 5) [12].
Figure 5: Reagents and conditions: (i) 2% H3PW12O40, EtOH, 120°C, microwave, 3 h, 68%. (ii) BrCCl3, DBU 2 h (quant.). (iii) Acetone, HCl 10% reflux, 1 h (95%). (iv) MeMgBr, THF, rt, 24 h, 30%. (v) Dess-Martin reagent, rt, 2 h, 94%. (vi) LiOH, THF/H2O, 24 h, rt, 65%. (vii) DCC, HOBT, DCM, Tryptamine, rt, 24 h, 50%.
In 2013, Davyt and co-workers described an 8-step procedure to Bacillamide C derivatives. Substituted nitrile is obtained by dehydration of amide, in which gaseous NH3 is not apt for the amplification. Thiazoline was synthesized from cysteine ester and then dehydrogenated by oxidation with a large amount of BrCCl3, the N-Boc protective group was removed and then acetylated, selectively hydrolyzed to give the substituted thiazolic acid. The final product was obtained by amide condensation after experiencing the above steps, it also required longer time for maintaining the necessarily low temperature [13]. In 2023, Jiang and co-workers modified the initial amidation using NH4HCO3, pyridine, (Boc)2O, 1,4-dioxane, however, other steps were remained without further optimizations (Figure 6) [14].
Figure 6: Reagents and conditions: (ia) NH3 (g), ClCO2Et, Et3N, THF, 10°C. (ib) TFAA, Py, THF, rt. 70% over 2 steps. (iia) cysteine ethylester hydrochloride, phosphate buffer pH 7, MeOH, 60°C. (iib) DBU, BrCCl3, CH2Cl2, 10°C. 66% over 2 steps. (iiia) TFA, CH2Cl2, rt. (iiib) Ac2O, Py, rt. 81% over 2 steps. (iv) 10% aqueous KOH, THF, rt, 80%. (v) tryptamine, HBTU, DIPEA, 0°C to rt, 29%.
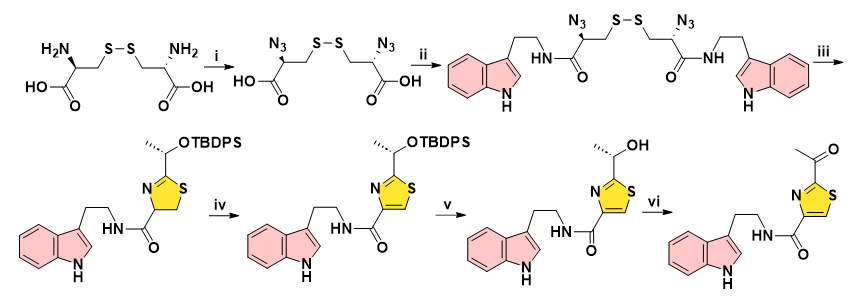
In 2013, Du and co-workers accomplished the synthesis of Bacillamides A and B in 30% overall yield for 6 steps. The starting L-Cystine underwent a one-pot four-step process of thiazoline formation via a cascade disulfide cleavage/thiocarbonylation/Staudinger reduction/aza-Wittig reaction, by the aid of β-azido disulfide and carboxylic acid as main substrates. TfN3 azides have certain safety hazards, while 8-equivalent PPh3, 5-equivalent BrCCl3 and 1.5-equivalent dangerous Dess-Martin reagents were employed (Figure 7) [15].
Figure 7: Reagents and conditions: (i) TfN3, CuSO4, K2CO3, 83%. (ii)Tryptamine, DCC, HOBt, rt, 5 h, 85%. (iii) EDCI (4.0 eq), DIPEA (8.0 eq), (S)-2-(tert-butyldiphenylsilyloxy) lactic acid (4.0 eq) in DCM, 15 min, then PPh3 (8.0 eq), reflux, 24 h, 75%. (iv) DBU (5.0 eq), BrCCl3 (5.0 eq), DCM, rt, 4 h, 67%. (v) TBAF (1.5 eq), THF, rt, 15 min, 85%. (vi) Dess-Martin reagent (1.5 eq), DCM, rt, 30 min, 90%.
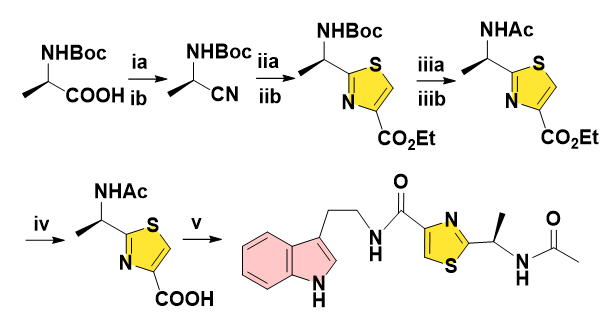
In 2018, Xu and co-workers designed a 5-step sketch utilizing N-Boc-alanine to synthesize Bacillamide D. The thionation by Lawesson’s reagent in classical Hantzsch reaction was modified in the presence of P2S5 for the convenience of one-pot scale-up mode with facile isolation and purification. However, the large amount of P2S5 and bromopyruvate as an irritant, volatile and polluting reagent could not be replaced (Figure 8) [16,17].
Figure 8: Reagents and conditions: (ia) aq. NaOH, 0°C, (Boc)2O, then rt 8 h. (ib) (Boc)2O, pyridine, NH4HCO3, rt 12 h, 92% for 2 steps. (ii) Na2SO4, DME, P2S5, ethyl 3-bromopyruvate, 85%. (iii) LiOH, THF/MeOH/H2O, 87%. (iva) iso-butyl chloroformate, NMM, DCM. (ivb) tryptamine, NMM, DCM. 57% for 2 steps. (v) 3 N HCl, ethyl acetate, 85%.
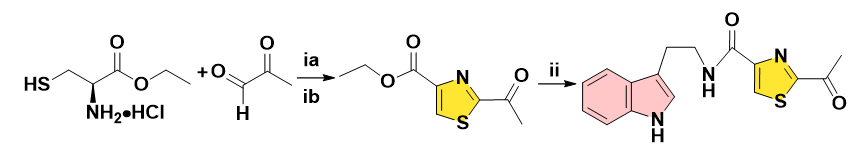
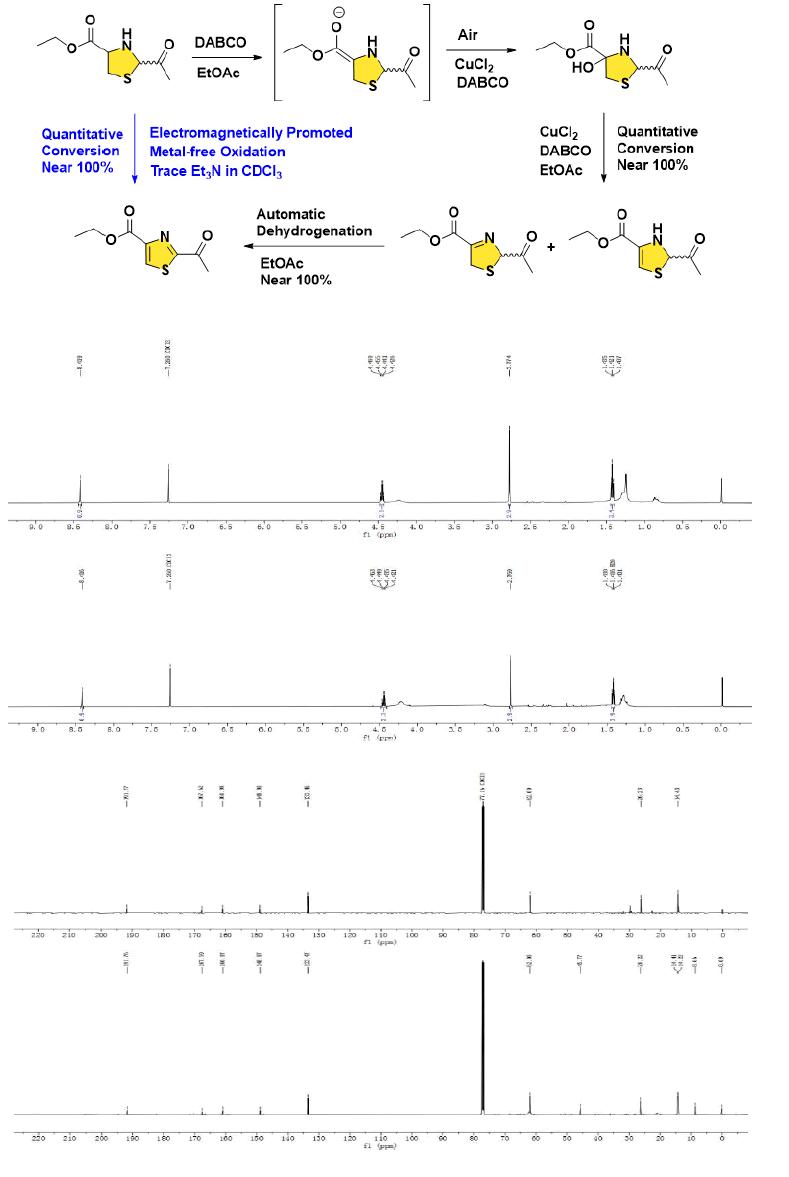
In 2018, Aggarwal and co-workers performed the synthesis of Bacillamide A from L-cysteine ethyl ester hydrochloride through an efficient and convergent approach. Iodobenzene diacetate, a versatile oxidising agent was chosen to furnish the substituted thiazole ester, the following solvent-free aminolysis of esters could achieve the title amide. During the procedures, the 2-equivalent organic hypervalent iodine brings safety hazards. To address this challenging issue, herein, we supplied one alternative strategy after comprehensively screening a series of oxidising combinations. Under the assistance of air as one green oxidant, the full conversion was accomplished via the adoption of the ratio of thiazolidine (one-pot)/CuCl2/DABCO=1/0.1/0.25 in EtOAc at 80°C for 2 h, the reactants in the EtOAc were simply purified by H2O washing, a nearly quantitative yield with high purity was verified by the NMR spectrum of the crude product (Figure 9) [18].
Figure 9: Reagents and conditions: (ia) NaHCO3, EtOH-H2O, 60ºC, 6-8 h. (ib) then IBD, DCM, 40ºC, 3-4 h, 62% for one-pot 2 steps. (ii) Tryptamine, 4,6-dimethylpyrimidin-2-ol, 100ºC, 6 h, 82%.
The possible mechanism was proposed on the basis of relational references, the initial enolization of thiazolidine was followed by installation of an angular hydroxide, which was oxidized either by a copper oxo species or dioxygen itself under the catalysis of CuCl2/DABCO [19]. After dehydration to form unsaturated thiazoline and/or its isomer, the automatic dehydrogenation from thiazoline to thiazole was facilitated [20]. To our astonishment, the nearly full conversion from the crude thiazolidine to the thiazole directly was also realized, through an electromagnetic wave/field driven mode in the presence of trace amount of Et3N in CDCl3, when the thiazolidine sample was checked for relating spectra on Bruker BioSpin GmbH (500 MHz), however, this transformation could not be repeated under air-free or air-exposed conditions, at r.t. or with conventional heating up to 70ºC, further investigation focusing on the intriguing mechanism is underway in our labs (Figure 10).
Figure 10: The proposed mechanism for dehydration and oxidation to generate bis-substituted thiazole along with the crude NMR spectra (CuCl2/DABCO/EtOAc system, upper; Et3N/CDCl3 electromagnetically driven pattern, below; trace amount of solvent residues may exist).
CONCLUSION
In summary, a variety of synthesis routes to the family members of bacillamide was compared with short comments, those different ideas being converged inside inspired us to successfully achieve a practical and scalable synthesis of key ester intermediate for the typical alkaloids. This biomimetic preparation and environmental benign technology provide alternative choices for building versatile libraries, which could be conveniently implemented in mass application of multi-functionalized candidates.
ACKNOWLEDGEMENT
The authors gratefully thank the following financial supports provided by the Natural Science Foundation of Guangxi (2020GXNSFGA297002, 2020GXNSFFA297005), the Special Fund for Bagui Scholars of Guangxi (05019055) and the Development Program of High-level Talent Team under Qihuang Project of GXUCM (No. 2021004).
References
- Gallardo?Rodríguez JJ, et al. Rev Aquacult. 2019;11(3):661-684.
- Anantapantula SS, et al. Water Res. 2023;243:120342.
[Crossref] [Google Scholar] [PubMed]
- Moreno-Andrés J, et al. J Hazard Mater. 2023;452:131279.
[Crossref] [Google Scholar] [PubMed]
- Lee S, et al. Applied Environ Microb. 2000;66(10):4334-4339.
[Crossref] [Google Scholar] [PubMed]
- Jeong SY, et al. Tetrahedron Lett. 2003;44(43):8005-8007.
- Socha AM, et al. J Nat Prod. 2007;70(11):1793-1795.
[Crossref] [Google Scholar] [PubMed]
- Zhang F, et al. J Biotechnol. 2019;292:5-11.
[Crossref] [Google Scholar] [PubMed]
- Fortinez CM, et al. Nat Commun. 2022;13(1):548.
[Crossref] [Google Scholar] [PubMed]
- Figueira VBC, et al. Arkivoc. 2005;14:14-19.
- Wang W, et al. Org Biomol Chem. 2010;8(3):529-532.
[Crossref] [Google Scholar] [PubMed]
- Bray CD, et al. Synlett. 2010;2010(4):599-601.
- Fache F, et al. Synth Commun. 2012;42(14):2098-2109.
- Martínez V, et al. Tetrahedron Asym. 2013;24(24):1572-1575.
- Wang J, et al. J Ocean Univ China. 2023;22(3):790-800.
- Sun X, et al. Organ Biomol Chem. 2015;13(14):4271-4277.
[Crossref] [Google Scholar] [PubMed]
- Wang B, et al. Mar Drugs. 2017;15(8):247.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, et al. Sci Rep. 2018;8(1):8555.
[Crossref] [Google Scholar] [PubMed]
- Kumara S, et al. Arkivoc. 2018;43(1):354-361.
- Dawsey AC, et al. Dalton Trans. 2012;41(26):7994-8002.
[Crossref] [Google Scholar] [PubMed]
- Lu Yet al. J Med Chem. 2009;52(6):1701-1711.
[Crossref] [Google Scholar] [PubMed]