Original Articles: 2021 Vol: 13 Issue: 2
Phenotypical, Mycochemicals, Proximate Composition and Antifungal Activity of Phylloporia ribis (Schumach) Ryvarden from India
Talluri Ribka1, Praveen Kumar Nagadesi2*, Vasavi Ponnuru1, Vijayadurga Thatha3 and Akella V.K.S.N. Pratyusha4
1 Department of Botany, Post Graduate Wing, Andhra Loyola College, Vijayawada, Andhra Pradesh, India
2 Department of Botany, St Joseph’s College, Lalbagh road, Bangalore, Karnataka, India
3 Department of Botany, C. R. Reddy College for Women's, Vatluru, Andhra Pradesh, India
4 Department of Chemistry, Post Graduate wing, Andhra Loyola College, Vijayawada, Andhra Pradesh, India
Abstract
Herbal medicines were popular in the treatment of many diseases due to green medicine is safe, easily available and less side effects. So the mushrooms belonging to Hymenochaetace members like Phellinus and Phylloporia were used in preparation of crude drugs/folk medicines. The sporophores of Phylloporia members were collected from Andhra Pradesh, India. The sporophore was used for phenotipical identification, Mycochemical evaluation, physicochemical properties, and Antifungal activity against plant pathogens. Based on the phenotypical or morphological characters the sporophore was identified as Phylloporia ribis (Schumach.) Ryv.
The extracts of P. ribis sporophore powder contain Carbohydrates, Proteins, Amino acids, Lipids, Alkaloids, Glycosides, Cardiac glycerides, Flavonoids, Phenols, Terpinoids, Steroids, Sterols, saponins, Tannins, and Phosphate. The best solvent for extraction is methanol when compared to ethanol and water. The wide range of bioactive compounds constituents would be useful for immunity boosters as food supplements, drug discovery and development of various new formulations. The proximate composition evaluation is useful for standardization of P. ribis in powder form. That will help to identify the genuine specie in adulteration test. The methanolic extract of p. ribis sporophore powder shown best antifungal activity when compared to water extract. Except 5% concentration all methanolic extracts of P. ribis shown 100% inhibition of Aspergillus niger causing soft rot of carrot. For the first time Mycochemical bio active compounds, proximate composition evaluation, antifungal activity of P. ribis were reported from India.
Keywords
Mycochemical; Immunity boosters; Antifungal; Phylloporia ribis; Nutritional value
Introduction
Medicinal mushrooms have been proposed as a novel therapy for several diseases for the survival of patients. So, mushrooms contain powerful compounds that enhance and balance your body’s ability to fight disease and stay healthy. They have been used medicinally since at least 3000 BCE. Eastern medical practitioners have known for more than 5,000 years that mushrooms contain powerful immune-boosting compounds and protective properties. Mushrooms are reported to have antimicrobial, anti-inflammatory, cardiovascular-protective, antidiabetic, hepatoprotective, and anticancer properties. It is well-established that mushrooms are adept at immune modulation and affect hematopoietic stem cells, lymphocytes, macrophages, T cells, dendritic cells (DCs), and natural killer (NK) cells [1]. Hymenochaetaceae have genus like Phellinus Quél. (1886:172), Inonotus P. Karst. (1879:39), Coltricia Gray (1821:644), Phylloporia Murrill (1904:141) and Cyclomyces Kunze ex Fr. (1830:512) [2]. The species diversity of genus Phylloporia was well-studied worldwide. It is widespread in European forest regions, North America forest, and Asia forests [3]. Phylloporia Murrill was introduced in the Hymenochaetaceae Donk, as an unusual polypore species. P. parasitica Murrill growing on the underside of living leaves in Columbia was studied by Murrill [4]. The species concept in Hymenochaetaceae family with Hymenomycetes genus was studied by Parmasto [5]. The taxonomy of several pairs of closely related Hymenomycetes species like Phylloporia ribis and P. ephedrae was described and A new combination of P. ephedrae (Voronich.) Parm was proposed as new taxa [5]. Basidiocarps of Phylloporia are used as a source of natural medicine in China [6]. In China P. ribis is used as edible fungus [6]. It was found that fruiting bodies of P. ribis have been used as food and functional ingredients for the treatment of pharyngitis, laryngitis, tonsillitis, and hyperglycemia [7]. But in India the preliminary work on medicinal mushroom like P. ribis was done. So in the present study aims at Mycochemical bioactive compounds, proximate composition and antifungal activity of P. ribis sporophore powder were tested.
Experimental Section
Collection and Phenotypical Identification
The sporophore was collected from Andhra Pradesh, India, during the rainy season (July-September) of the years 2017 to 2019. Field characters like habit, host, name of locality and other macro-morphological characteristics were recorded for sample specimens. For Phenotipical identification of sporophore, different Macroscopic features like abhymenial, hymenial surfaces, context, and pore tubes of species were examined. Microscopic features like hyphae, basidiospores and pilear crust were observed by preparing crush mounts and free-hand sections in water, 5% KOH solution, and staining was done with cotton blue (1%, in lacto phenol), Congo red (1%, in distilled water), phloxine (1%, in distilled water), and Melzer’s reagent [8-12]. Voucher specimen of P. ribis (ALC 30) has been deposited at the herbarium of the Museum of Botany Department, Andhra Loyola College, Vijayawada, Andhra Pradesh, India (ALC).
Extraction of Bioactive Compounds
The sporophore of P. ribis was initially rinsed thrice in distilled water and dried on paper toweling and samples was cut into fine pieces and powdered. For preparing the extracts methanol, ethanol and water was used as solvents. For every 1 gram of powder, 50 ml of solvent was used and was subjected to extraction using maceration. After the completion of extraction, the supernatant was filtered through Whatman No. 1 filter paper and the filtrates stored at 4°C for further use to perform various assays for determination of bioactive mycochemicals and antifungal activity.
Mycochemical Tests
The screening of bioactive mycochemicals in fresh sporophores of P. ribis is tested by using standard methods of Indian Pharmacopoeia followed by [13-19].
Test for Carbohydrates
Molisch’s test: To a small amount of the extract few drops of Molisch’s reagent was added followed by the addition of conc. H2SO4 along the sides of the test tube. The mixture was then allowed to stand for 2 min and then diluted with 5 ml of distilled water. Formation of red or dull violet colour at the inter phase of two layers indicates the presence of carbohydrates. First yellow then brick red precipitate was observed.
Fehling’s test: The extract was treated with 5 ml of Fehling’s solution (A and B) and kept in boiling water bath for 5-10 min. The formation of yellow or red colour precipitate indicates the presence of reducing sugar
Test for Proteins
Biuret test: Test sample (3 ml) was mixed with 4% NaOH and few drops of 1% CuSO4 solution were added. Violet or pink color not appeared. To 3 ml of the extract few drops of 10% sodium chloride and 1% copper sulphate was added for the formation of violet or purple colour. On addition of alkali, it becomes dark violet.
Tests for Amino Acids
Ninhydrin test: Test sample (3 ml) and 3 drops of 5% ninhydrin solution were heated in boiling water for 10 mins. Purple color appeared.
Test for Lipids
Brown bag test: Certain kinds of paper such as a piece of brown paper bag can readily absorb lipids and can be used to test for the presence of lipids.
Emulsion test: Suspended the sample in ethanol which allows lipids to dissolve in it, and then decanted the liquid into water. Since lipids do not dissolve in water, it falls out of the solution as cloudy white emulsion.
Test for Glycosides
Free content of the sugar extract was determined. The sample was hydrolysed with mineral acid (dilute hydrochloric or dilute sulphuric acid). Again the total sugar content of the hydrolysed extract was determined. Increase in the sugar content indicated the presence of glycoside in the extract.
Glycoside+H2O→ Aglycon (genin)+Glycon (sugar)
Liebermann’s test: We added 2.0 ml of acetic acid and 2 ml of chloroform with whole aqueous plant crude extract. The mixture was then cooled and we added H2SO4 concentrated. Green color showed the entity of aglycone, steroidal part of glycosides.
Legal’s test: Aqueous or alcoholic sample extract was mixed with 1 ml of pyridine sodium nitroprusside was added. Pink to red color appeared.
Test for Cardiac Glycosides
Keller-Kiliani test: A solution of glacial acetic acid (4.0 ml) with 1 drop of 2.0% FeCl3 mixture was mixed with the 10 ml aqueous plant extract and 1 ml H2SO4 concentrated. A brown ring formed between the layers which showed the entity of cardiac glycosides.
Test for Steroids
Salkowski’s test: Sample: Sample (2 ml) was mixed with 2 ml of concentrated Sulphuric acid, it was well shaken then chloroform layer appeared red and acid layer shown greenish yellow fluorescence.
Lieberman-Buchard reaction: Sample (2 ml) was mixed with chloroform. 1-2 ml of acetic anhydride was added and 2 drops concentration sulphuric acid was added from the sides of the tube. First red then blue and finally green colour appeared.
Tests for Sterols
The sample was treated with 5% potassium hydroxide solution appearance of pink colour indicated the presence of sterols.
Test for Saponin
Foam test: To 1 ml of the extracts 5 ml distilled water was added and shaken vigorously. Formation of foam indicated presence of saponins.
Phosphate Test
Ammonium molybdate test: A small amount of the sample is acidified with concentrated nitric acid, to which a little ammonium molybdate is added. The presence of phosphate ions is indicated by the formation of a bright yellow precipitate layer of ammonium phosphomolybdate. The appearance of the precipitate can be facilitated by gentle heating
Silver nitrate test: To a small amount of sample add silver nitrate solution. Silver phosphate is formed as a yellow precipitate by the reaction between a soluble silver compounds, such as silver nitrate with a soluble orthophosphate.
Proximate composition: To establish standards for their identity, quality, and purity of hymenophore powder the proximate composition was carried out for P. ribis collected from ALC, Vijayawada, Andhra Pradesh, India. The pulverized sporophore of P. ribis was used for the standardization of physicochemical parameters in triplicate. Foreign matter, moisture content, extractive values, ash values [20,21] dry matter [22], absorption properties, foaming properties [23], emulsion values [24], dispersibility [25], flow characteristics, swelling index [26] were determined.
Antifungal Test
To test the antifungal activity of P. ribis, the method described by Nagadesi and Arya [27] was used. The Aspergillus niger was causing soft rot of carrot, A. oryzae was causing mold in paddy seeds, Mucor racemosus causing soft rot if bitter guard, Rhizopus stolonifer was causing soft rot in lady’s finger and R. artocarpi causing fruit rot in Jack fruit was isolated and used for antifungal test. The fungal extracts were mixed with appropriate volume of medium (PDA) to obtain concentrations ranging from 5 to 25% in the final volume of 100 ml of medium. This 100 ml medium was dispensed into 100 mm Petri plates with triplicates. Plant pathogenic fungi were placed in the centre of each plate. Control sets were also prepared without fungal extract. The plates were incubated at 25 ± 20°C and growth of colony was measured after 7 days of inoculation. The radial growth of mycelium was measured at two points along the diameter of the plate and mean of these two readings was taken as the diameter of the colony. The growth of colony in control sets was compared with that of various treatments and difference was converted into percent inhibition by following formula

Results and Discussion
Phenotypical Identification
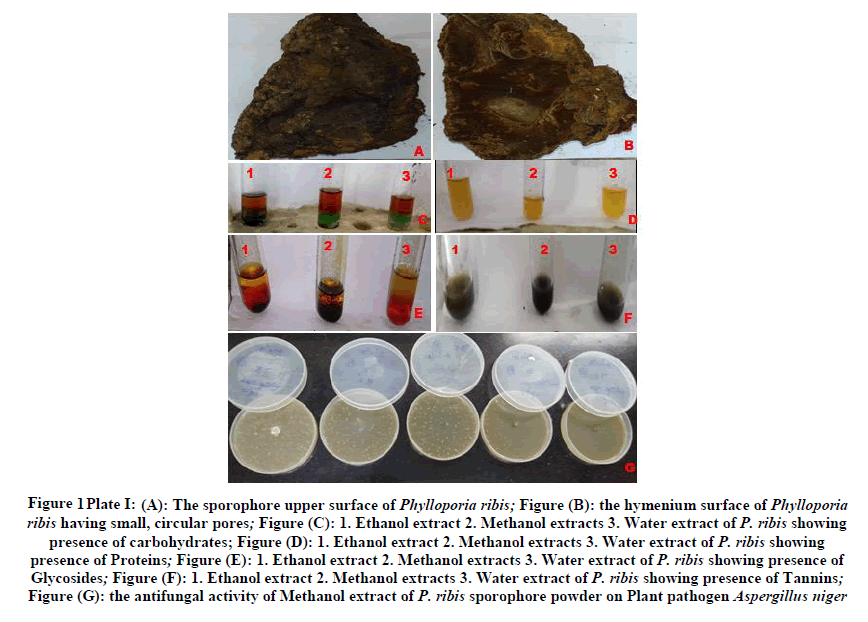
The sporophore was found on the living tree trunk of Peltophorum roxburghii (G.Don) Degener causing bunt rot. The sporophore is identified as P. ribis (Schumach.) Ryvarden, belonging to Hymenochaetaceae family. The description of the sporophore was given below Phylloporia ribis (Schumach.) Ryvarden, Basidiocarps perennial, sessile, woody, semicircular often encircling the stem on which it is growing, often several basidiocarps fused to become imbricate with overlapping pilei, pilei flat and up to 21.4 × 13.2 × 11,5 cm in size; upper surface is sulcate, concentric bands with depressions, dark brown when young, black when it becomes old, smoother with pits and small tubercles, soft and compressible, in actively growing basidiocarps have yellowish brown wavy margin, the upper part distinctly separated from the context proper by a black zone (Plate I Figure 1(A)); Hymenium surface dark rusty brown, pores small and circular,7-9 per mm, invisible to the naked eye; tube layers concolorous, up to 1 cm thick and indistinctly stratified (Plate I Figure 1(B)); Context dense and shiny when broken, dark reddish brown, thicker than the upper soft tomentum which is separated by a black zone, in compound basidiocarps this black zone may occur irregularly in the context due to late fusion of previous separate basidiocarps. Hyphal system monomitic; generative hyphae with simple septa, thick-walled, in the tomentum sparingly branched with scattered septa, yellow to pale rusty brown, 3.25-6.5 μm in diameter, in the context and trama golden brown coloured and up to 9,7 μm in diameter, in the subhymenium hyaline and thin-walled, 3.25 μm in diameter.
Figure 1: (A): The sporophore upper surface of Phylloporia ribis; Figure (B): the hymenium surface of Phylloporia ribis having small, circular pores; Figure (C): 1. Ethanol extract 2. Methanol extracts 3. Water extract of P. ribis showing presence of carbohydrates; Figure (D): 1. Ethanol extract 2. Methanol extracts 3. Water extract of P. ribis showing presence of Proteins; Figure (E): 1. Ethanol extract 2. Methanol extracts 3. Water extract of P. ribis showing presence of Glycosides; Figure (F): 1. Ethanol extract 2. Methanol extracts 3. Water extract of P. ribis showing presence of Tannins; Figure (G): the antifungal activity of Methanol extract of P. ribis sporophore powder on Plant pathogen Aspergillus niger
Setae in hymenium were absent, Basidia clavate, 4-sterigmate, 12.5-16.6 × 6.5-7.7 μm simple-septate at the base. Basidiospores usually abundantly present, ellipsoid, thin-walled, pale yellow to hyaline, negative in Melzer's reagent, 3-4.5 × 2.5-3 μm.
Habitat: Found on the living tree trunk at the base of Peltophorum rouxbergii causing white rot, from Andhra Loyola College, Krishna district, Andhra Pradesh, India, collected by N. Praveen Kumar, Accession no: ALC 30. 20-8-2017.
Phylloporia species are mostly parasitic on lianas and roots of shrubs and trees, which are difficult to identify in the Neotropics. As a result, the extent of host specialization of Phylloporia species is unknown [28]. In the present study the P. ribis is causing bunt rot at the base of the living tree. P. ribis was reported growing strictly on the roots of living Crataegus species of Ribes, Lonicera and Symphoricarpos, which could produce white rot and heartrot in living hardwoods [29]. In the present study the P. ribis is found on living tree trunk at the base of P. rouxbergii, which causing white rot. P ribis. It is distributed throughout East Asia also and, is a white-rot fungus that prefers to live on stumps of Rosa polyantha and Weigela subsessilis [30]. In the present study the P. ribis was reported from Andhra Pradesh, India.
Mycochemical Bioactive Compounds
The mycochemical screening of three different extracts showed great variation in terms of bioactive compounds (Table 1).
| S.No | Mycochemicals | Mycochemical test | Ethanol | Methanol | Water |
|---|---|---|---|---|---|
| 1 | Carbohydrates | Molisch’s test | +++ | ++++ | +++ |
| Fehling’s test | +++ | +++ | ++ | ||
| 2 | Proteins | Biuret test | +++ | +++ | +++ |
| 3 | Amino acids | Ninhydrine test | +++ | ++ | ++ |
| 4 | Lipids | Brown Bag test | ++ | +++ | + |
| Emulsion test | ++ | + | -- | ||
| 5 | Alkaloids | Wagner’s test | - | +++ | ++ |
| Mayers test | - | + | + | ||
| 6 | Glycosides | Liebermann’s Test | - | +++ | ++ |
| Legal’s test | - | ++ | -- | ||
| 7 | Cardiac glycerides | Keller-Kiliani Test. | +++ | ++ | ++ |
| 8 | Flavonoids | Lead acetate solution test | ++++ | ++++ | ++ |
| 9 | Phenols | Ferric chloride test | ++ | +++ | -- |
| 10 | Terpinoids | Saloweski test | +++ | ++ | ++ |
| 11 | Steroids | Lieberman-Buchard reaction | ++ | +++ | + |
| Salkowski’s test | ++ | ++++ | + | ||
| 12 | Sterols | KOH test | + | ++ | + |
| 13 | Saponins | Forthing Test | + | +++ | ++ |
| 14 | Tannins | Ferric chloride test | - | +++ | ++ |
| 15 | Phosphates | Ammonium molybdate test | +++ | +++ | ++ |
| Silver nitrate test | + | ++ | ++ |
+=Present, ++=moderately present, +++ (or) ++++=Excellent
Table 1: Mycochemical composition of Phylloporia ribis in different solvent extracts
The extracts of P. ribis contain Carbohydrates, Proteins, Amino acids, Lipids, Alkaloids, Glycosides, Cardiac glycerides, Flavonoids, Phenols, Terpinoids, Steroids, Sterols, saponins, Tannins, and Phosphate.
The best solvent for extraction is methanol when compared to ethanoland water. The methanolic extract of P. ribis showed excellent concentration of Carbohydrates, Proteins, Lipids, Alkaloids, Glycosides, Flavonoids, Phenols, Steroids, saponins, Tannins, and Phosphate. The ethanolic extract of P. ribis showed excellent concentration of Carbohydrates, Proteins, Amino acids, Cardiac glycerides, Flavonoids, Terpinoids. The water extract of P. ribis showed the excellent concentration of Carbohydrates, Proteins (Plate I Figure (1C-1E)).
The optimum polysaccharide extraction method of P. ribis (Schumach.:Fr.) Ryvarden (PrR) was studied and also compared the polysaccharide content of PrR collected in different years The polysaccharide content of annual and biennial PrR was 4.48% and 4.01% respectively. It is concluded that content of PrR polysaccharide decreased with the increase of years [31]. In the present study the P. ribis shown excellent concentration of carbohydrates and proteins. A comprehensive screening shows that ergothioneine is the most abundant antioxidant in the wild macrofungus P. ribis Ryvarden [32].
In the present study the P. ribis showed the antioxidant compounds like Phenols flavonoids terpinoids. The effect of P. ribis glucan (PRG) administration against immune injury due to free radical formation was evaluated in mice. The results showed that glucan administration significantly increased thymus and spleen indices, spleen lymphocyte proliferation and NK cells activity, as well as CD8 T cell numbers, and decreased CD4+CD8+[33]. In the present study the P. ribis had shown excellent concentration of carbohydreates proteins glycosides and phenols so this should be useful for immunomodulator.
Proximate Composition
The hymenophore powder of P. ribis shows the proximate composition in Table 2. Foreign matter present in sporophore powder of P. ribis is 0.5% whereas moisture content in sporophore powder of P. ribis is 7.5%. The higher the dry matter in sporophore of P. ribis indicates presence of less moisture. The extractive value of P. ribis powder shows higher ethanol soluble value when compared to water soluble content. The ash content of P. ribis sporophore powder is 4.89%, showing higher acid insoluble ash when compared to water soluble ash. The absorption capacity of P. ribis sporophore powder shown higher water absorption when compared to oil absorption. The emulsion formation capacity of P. ribis sporophore powder shown low dispersibility when compared to emulsion stability. The flow properties of P. ribis sporophore powder of P. ribis shown tapped density is more when compared to bulks density. The foam formation capacity of P. ribis sporophore powder shown higher swelling capacity when compared to foaming capacity.
| S. No | Parameter | Properties | Phylloporia ribis |
|---|---|---|---|
| 1 | Physical parameters | Foreign matter (%) | 0.5 |
| 2 | Moisture content (%) | 7.5 | |
| 3 | Dry matter (%) | 82.9 | |
| 4 | Extractive values (%) | Ethanol soluble extractives | 2.25 |
| Water soluble extractives | 1.87 | ||
| 5 | Ash content (%) | Total ash | 4.89 |
| Acid insoluble ash | 1.56 | ||
| Water soluble ash | 1.21 | ||
| 6 | Absorption properties (ml/g) | Oil absorption capacity | 5.98 |
| Water absorption capacity | 48.56 | ||
| 7 | Emulsion properties (%) | Emulsifying capacity | 34.45 |
| Emulsion stability | 25.43 | ||
| Dispersibility (%) | 20 | ||
| 8 | Flow properties | Bulk density (g/ml) | 0.56 |
| Tapped density (g/ml) | 0.78 | ||
| 9 | Foaming properties (%) | Foaming capacity | 15.67 |
| Foaming stability | Oct-25 | ||
| Swelling Index (%) | 50 |
Table 2: Proximate composite evaluation in sporophore of Phylloporia ribis
The Phellinus pachyphloeus showed negligible foreign matter and low moisture content as 13.67% [34], in the present study the P. ribis showed very low amount of foreign matter and moisture content as 7.5%. It also shows high dry weight as 86.33% [34]. In the present study also the P. ribis showed high dry weight as 82.9%. It also shown good flow characteristics and high dispersibility (85.67%) [34]. In the present study, the P. ribis shown moderate flow characteristics and low dispersibility. It also has shown high alcohol soluble extractives [34]. In the present study also high alcohol soluble extractives was observed. It also has shown high water absorption capacity [34]. In the present study the P. ribis shown high water absorption capacity. It also had shown good emulsion properties [34]. In the present study also the P. ribis shown high emulsion character. It also shown high content of water soluble ash (3%) [34] In the present study the P. ribis shown low water soluble ash content.
Antifungal Activity
The antifungal activity of P. ribis on plant pathogenic fungi was shown in Table 3. The methanolic extract of P. ribis shown best antifungal activity when compared to water extract. The 20%, 25% concentration of methanolic extract of P. ribis shown 100% inhibition of plant pathogenic fungi tested. The 25% concentration of water extract of P. ribis has shown 100% inhibition of plant pathogenic fungi. Except 5% concentration all methanolic extracts of P. ribis shown 100% inhibition of Aspergillus niger (Plate I Figure 1(G)).
| Fungi | Methanol | Water | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 5% | 10% | 15% | 20% | 25% | 5% | 10% | 15% | 20% | 25% | |
| Aspergillus niger | 86.1 | 100 | 100 | 100 | 100 | 45.4 | 62.3 | 73.5 | 91.3 | 100 |
| A. oryzae | 89.5 | 94.6 | 100 | 100 | 100 | 59.5 | 68.6 | 83.6 | 93.7 | 100 |
| Mucor racemosus | 78.3 | 92.2 | 100 | 100 | 100 | 38.3 | 57.2 | 72.5 | 89.6 | 99.2 |
| Rhizopus stolonifer | 87.6 | 96.2 | 99.3 | 100 | 100 | 43.8 | 60.6 | 76.9 | 93.5 | 100 |
| Rhizopus artocarpi | 83.8 | 94.9 | 100 | 100 | 100 | 60.5 | 70.2 | 83.4 | 90.1 | 98.9 |
Table 3: Antifungal activity of Phylloporia ribis in different plant pathogenic fungi
The live-cell filtrates of T. viride producing CuNPs shown better controlling on plant pathogenic fungi tested when compared to live-cell filtrates of A. niger. The 20% and 25% concentrations of a bio-controlling agent like A. niger and T.viride filtrates showed 100% inhibition of plant pathogenic fungi [35].In the present study also the methanolic extract of P. ribis with 20% and 25% concentration shown 100% inhibition of plant pathogenic fungi.
Conclusion
The present study suggests that, the Hymenochaetaceae members are valuable source in herbal medicines. Green medicine is safe, easily available and less side effects, so the different sporophores powders were used in preparation of crude drugs/folk medicines. The India sporophores of Phylloporia ribis contain Carbohydrates, Proteins, Amino acids, Lipids, Secondary metabolites and Phosphate. The present of different Mycochemical bioactive components within the P. ribis suggest that it is edible, rich in nutrients and the secondary metabolites may be used in promoting the immunity through diet, drug discovery and development of various new formulations. In the present study, the P. ribis should be utilized as a suitable antifungal agent against plant pathogens causing soft rot in Vegetables and fruits. The proximate composition evaluation is useful for standardization of P. ribis in powder form. That will help to identify the genuine specie in adulteration test. For the first time mycochemical bio active compounds, proximate composition evaluation, antifungal activity of P. ribis were reported from India. The studies should be performed further in order to isolate the bioactive principles in pure form which were responsible for Immunity booster, drug development, treatment of different pestilence, antioxidant, anti-inflammatory, antibiotic nature and antimicrobial activity.
Acknowledgments
The authors are thankful to the Principal, Vice principal (P.G), Andhra Loyola college, Vijayawada-8, for laboratory facilities in Botany Department. Prof. Arun arya, Head Environmental Science, Faculty of Science, The Maharaja Sayajirao University of Baroda, vadodara, for identification conformation of fungi.
References
- MF Moradali; H Mostafavi; S Ghods; GA Hedjaroude. Int Immuno-pharmacol. 2007, 7(6), 701-724.
- ERD Santos; GL Robledo; NC Limajúnior; E Malosso; MA Reckl; TB Gibertoni. Phytotaxa. 2016.
- X Zhang; Y Dai. Flora fungorum sinicorum, Hymenochaetaceae. Science Press, Beijing, (in Chinese). 2005, 29, 1-205.
- WA Murrill. Torreya. 1904, 4, 141–142.
- E Parmasto. Indian Acad Sci (Plant Sci). 1985, 94(2, 3), 369-380
- J Jiang; L Zhou; S Liu; L Zhou; X Tian. Phytotaxa, 2020, 446(4), 209–219.
- Y Fan; M Chen; W Zhou; L Xu; L Lu. Current Research Situation of Phylloporia ribis and its Prospects of Application and Exploitation, 15, Liaoning University of TCM, 2013.
- A Arya; S Albert; PK Nagadesi. Journal of Mycolology & Plant Pathology. 2008, 38 (2), 221–226
- PK Nagadesi; A Arya. Mycosphere. 2012, 3(6), 997–1004.
- PK Nagadesi; J Bhavani; A Arya. Int Lett Nat Sci. 2014, 12(1), 55–69.
- PK Nagadesi; G Aravind; B Kannamba. Biological Forum An international Journal, 2016, 8(2), 240–246
- PK Nagadesi. Indian Phytopathology. 2018, 71(4), 589-597
- WC Evans; GE Trease. Pharmacognosy, 13, Bailliere Tindall, London, 1989.
- SB Gokhale; CK, Kokate; AP Purohit. Pharmacognosy. Nirali Prakshan, Pune, India, 1993; 1–50.
- WC Evans; GE Trease. Pharmacognosy, 13, Bailliere Tindall, London, 1989.
- SB Gokhale; CK, Kokate; AP Purohit. Pharmacognosy. Nirali Prakshan, Pune, India, 1993; 1–50.
- GE Trease; WCA Evans. Pharmacognosy, 14, WB Saunders, London, 1996; 13–53.
- P Kokate; AP Purohit; SB Gokhale. Pharmacognosy, 20, Nirali Publication, India, 2002.
- S Shanmugam; TS Kumar; KP Selvam. Laboratory hand book on biochemistry, PHI learning Private limited, New Delhi, India, 2010; 129-133.
- MD Gaithersburg. Official methods of analysis of the Association of Official Analysis Chemists, 17, AOAC International, 2000.
- JB Harborne. Phytochemical methods, 3, Chapman and Hall, London, 2005; 49-244
- K Kornerup; JH Waanscher. Metheun’s handbook of colors. 3, Metheun and Co. Ltd. London, 1978, 252.
- KR Khandelwal. Practical Pharmacognosy Techniques and Experiments, Nirali Publication, India, 2006
- MD Gaithersburg. Official methods of analysis of the Association of Official Analysis Chemists, 17, AOAC International, 2000
- DN Kulkarni. Journal of Food and Nutrition. 1991, 13, 322-323.
- Indian Pharmacopoeia Commission. Indian Pharmacopoeia. New Delhi, Government of India Press. 2007, 1.
- MO Aremu; O Olaofe; ET Akintayo. Journal of Food Technology. 2007, 5, 109-115
- K Yatsumatsu; K Sawuda; S Moritaka; M Miscki. Journal of Agaricultural and Biological Chemistry. 1972, 36, 719-726.
- DN Kulkarni. Journal of Food and Nutrition. 1991, 13, 322-323.
- UK Prodhan; KMDMR Linkon; MDF Al-Amin; MDJ Alam. Journal of Food and Agriculture. 2015, 27(7), 542-547.
- PK Nagadesi; A Arya. Lignicolous Macro Fungi from Gujarat, India. World Scientific News. 2016, 44, 206-223.
- L Ryvarden. Neotropical Polypores. Part 1. Synopsis Fungorum 19. Oslo, Fungiflora. 2004.
- L Ryvarden; R Gilbertson. European polypores. Part 2. Synopsis Fungorum. 1994, 7, 394-743.
- U Azeem; GS Dhingra; R Shri. Taxonomy, Physicochemical Properties and Mycochemical Composition of Wood Rotting Mushroom Phellinus Pachyphloeus (Pat.) Pat. RJLBPCS. 2018, 4(2), 1-16.
- M Kubo; Y Liu; M Ishida; K Harada; Y Fukuyama. Chem Pharm Bull. 2014, 62(1), 122-124.