Original Articles: 2022 Vol: 14 Issue: 7
Passiflora edulis Juice Effect on Lipid Content in Primary Human Colon Adenocarcinoma Cells (SW480) and Their Lymph Node Metastatic Derivatives (SW620)
Juan Camilo Guerrero Ospina*, Nasly Jimena Garay, Beatriz Restrepo, Nelsy Loango, MaríaElena Maldonado-Celis, Patricia Landázuri
Department of Pharmaceutical Science, University of Quindío, Armenia, Colombia
- Corresponding Author:
- Juan Camilo Guerrero Ospina
Department of Pharmaceutical Science,
University of Quindío, Armenia,
Colombia
Received: 20-May-2022, Manuscript No. JOCPR-22-64358; Editor assigned: 23-May-2022, PreQC No. JOCPR-22- 64358 (PQ); Reviewed: 06-Jun-2022, QC No. JOCPR-22-64358; Revised: 19-Jul-2022, Manuscript No. JOCPR-22- 64358(R); Published: 26-Jul-2022 DOI: 10.37532/0975-7384.2022.14(7).1-10
Abstract
Cancer cells modify their lipid metabolism to proliferate. The Passiflora edulis fruit juice (FruJu) contains phytochemicals with antitumor properties. This work studied the effect of FruJu on cell viability, cholesterol concentration, and intracellular triglycerides in SW480 and SW620 cells. The antiproliferative activity was studied through sulforhodamine B; lipids through Folch’s method. At 12 h, under the same culture conditions, the cell lines showed differences in their growth rate (p=0.0034). The FruJu at 39.6 μg/ml diminished cell viability: SW480 (45.6%) and SW620 (45.1%). Without exposure to the FruJu, the concentrations of cholesterol and triglycerides were significantly higher in SW480 (6.9 μg/mgPt and 8.3 μg/mgPt, respectively) compared with SW620 (4.9 μg/mgPt and 7.0 μg/mgPt, respectively) (p<0.0025 and p=0.0037). The FruJu at 39.6 μg/ml diminished cholesterol significantly (44.6%) and triglycerides (46.5%) in SW480; while in SW620, cholesterol content decreased 14.8% and increased triglycerides by 7%. In conclusion, the FruJu diminished the proliferation capacity and cholesterol concentration in SW480 and SW620. These findings suggest that inhibition of cell growth could be associated with the intracellular biosynthesis of cholesterol, but not of triglycerides, hence, it is possible that the FruJu has different action mechanisms on the metabolism of these lipids in colon adenocarcinoma cells and their metastatic derivatives.
Keywords
Cholesterol; Triglycerides; Cell proliferation; Hormetic dose-response
Introduction
Colorectal Cancer (CRC) is the third most common form of cancer globally. Its management requires surgery, chemotherapy, or both. Although chemotherapy has improved the management and survival of patients with CRC, the collateral effects and resistance to such has led to the search for alternative treatments or adjuvants. Within this context, natural products with medicinal or nutraceutical properties are gaining importance due to their low toxicity at concentrations compatible with human intake and to their capacity to produce lower collateral effects compared with chemical drugs. Medicinal plants provide great amounts of bioactive molecules, like phenolic acids, flavonoids, carotenoid anthocyanins with proven cytotoxic and apoptotic activity against various types of cancer, including colorectal cancer. Several of these compounds have been identified from extracts from leaves, fruits, and seeds from different varieties of P. edulis, also called yellow passion fruit. The variety Flavicarpa degenerer is the most popular in the Passifloraceae family in Latin American countries [1].
For P. edulis biological activities have been demonstrated, such as sedative, antihypertensive, lipid-lowering, antioxidant and antiproliferative; regarding its antitumor properties, the study by Ramírez et al., evaluated the effect of an ethanolic extract of P. edulis leaves on colon adenocarcinoma cells (Caco-2), SW480 and their metastatic derivative SW620 and the non-tumor cell line CHO-K1 and found antiproliferative effect with a median Lethal Dose (LD50) of 167.62 μg/ml at 24 h; the study also evidenced an increased number of necrotic cells in cultures treated during 48 h and increased SubG1 and G2/M populations. Moreover, the authors increased caspase-3 activity and demonstrated accumulation of the oxidized glutathione with greater selectivity for SW620 cells and lower in CHO-K1 cells additionally, the study by Aguillón et al., with P. edulis juice in SW480 and SW620 cells, found an antiproliferative effect with IC50 444 mg/ml and 415 mg/ml, respectively. Exposure to the juice reduced the growth of the SW480 and SW620 cells by 40% and 50%, respectively. Data from both studies evidence an anticancer potential of P. edulis, which seems to be mediated among other mechanisms, for affecting the cell cycle, as indicated in the findings by Ramírez et al., by showing that the treatment with the P. edulis ethanolic extract increases the subG1 and G2/M subpopulations, phases during which cells reorganize and replenish nutrients, including lipids; not going to the following phases when exposed to the P. edulis extract could suggest lower supply of nutrients, like triglycerides and cholesterol, by decrease in their synthesis or uptake, given that these compounds are important energy sources, besides being structural components of the cell membranes and playing an important role in the cellular signaling pathways. Gathered evidence indicates that cancer cells activate synthesis and uptake of fatty acids and cholesterol, activating multiple oncogenic pathways that converge on fatty acid synthesis, like the PI3K/Akt pathway. Changes in lipid metabolism can affect numerous cell processes, including growth, proliferation, differentiation, invasion and metastasis thereby, the aim of this work was to investigate the effect of the P edulis juice (FruJu) on cell viability, intracellular cholesterol and triglyceride content in in primary human colon adenocarcinoma cells (SW480) and their metastatic derivatives in lymph nodes (SW620) [2].
Materials And Methods
Methodology
Plant material and preparation of the Passiflora edulis FruJu: The healthy and ripe P. edulis fruits var. flavicarpa degenerer were picked between 6:00 am and 8:00 am in the municipality of La Tebaida, department of quindío in Colombia. Fruit ripeness was determined through its color and by the maturity index (°brix/acidity), according with the Colombian technical norm NTC1267, which establishes the requisites that need to be complied by the P. edulis fruit for its fresh consumption. The plant was identified by the herbarium at Universidad del Quindío (H18063). The FruJu was extracted through mechanical methods (not including the seed) and then lyophilized and stored at -20°C until use. For cell viability, four concentrations were evaluated: 39.6, 52.8, 66.0, 132.0 μg/ml of the lyophilized diluted in the culture medium [3].
Physicochemical, phytochemical characterization and antioxidant activity of the P. edulis FruJu
Total phenol content: Total phenol content was determined according to the modified Folin-Ciocalteu method The FruJu (10 μl) was mixed with 125 μl of Folin-Ciocalteu reagent and 400 μl of sodium carbonate solution (7.1% p/v); the resulting solution was taken to a final volume of 1000 μl. The mixture was incubated at room temperature during 30 min in darkness. Absorbance was measured at 760 nm against a target. A standard solution of Gallic acid was used to conduct the calibration curves. Results were expressed as mg of Gallic Acid Equivalent (GAE)/l.
Total flavonoid content: Flavonoids were determined through the colorimetric method described by Marinova et al., The procedure mixed 100 μl of FruJu with 30 μl of NaNO2 (5% p/v), 30 μl of AlCl3 (10% p/v), 200 μl of NaOH (1M), and the resulting solution was taken to a final volume of 1000 μl with distilled water. Absorbance was measured at 510 nm. The standard solution of (+)-catechin was used to carryout the calibration curves and results were expressed as catechin/L equivalents [4-6].
Determination of monomeric anthocyanins: The differential pH method described by Giusti and Wrolstad was used. The D.O. was measured in a spectrophotometer (Beckman Instruments, Inc., Fullerton, CA) at 530 and 700 nm, using pH 1.0 and 4.5 buffers, estimation of total monomeric Anthocyanins (AT) was conducted by using the expression: AT (mg/100 mL)=ΔΑ PM FD 100/? 1 where: ΔA=((A530-A700) pH1.0-(A530-A700) pH4.5), with a molar extinction coefficient (?) for cyanidin-3-glucoside (C-3-G) de 26900 and molecular weight (PM) for cyanidin-3-glucoside, 449.2 g/mol. Results were presented as milligram equivalents of cyanidin-3-glucoside (C3G) per 100 ml of sample (mgEq C3G/100 mL) ± SD [7-11].
Determination of the total tannin content: An aliquot of 250 μl of the mango extract was taken, adding 500 μl of Bovine Serum Albumin (BSA) solution in acetic acid buffer 0.2 M with pH 5.0, mixing carefully, and letting to rest for 15 min. It was then centrifuged at 5,000 g x for 15 min and the supernatant was discarded, the precipitate was diluted with 1 ml of Sodium Dodecyl Sulfate (SDS) solution at 1% and triethanolamine at 4%. Finally, 250 μl of Ferric Chloride (FeCl3) was added at 0.01 M in Hydrochloric acid (HCl) at 0.01 M and incubated for 30 min at RT. Absorbance was analyzed at 510 nm in spectrophotometer (BioTeck, Epoch microplate). As target, 1 ml of SDS solution was used at 1% and triethanolamine at 4% in 250 μl of FeCl3 solution at 0.01 M in HCl at 0.01 M. The results were expressed as mg of Tannic Acid Equivalents per gram of FruJu (mg TAE/g FruJu extract). Tannic acid was used as standard for the calibration curve [12-15].
Determination of the total alkaloid content: The bromocresol green method was used. Briefly, 1 ml of the P. edulis FruJu was mixed with 5 ml of phosphate buffer (pH 4.7) and 5 ml of bromocresol green solution, thereafter, adding 2 ml of chloroform this last step was repeated twice; lastly, absorbance of the extract collected was measured at 470 nm in spectrophotometer (BioTeck, Epoch microplate). Quinine was used as standard to elaborate the calibration curve. The results were expressed as mg of quinine equivalents per gram of FruJu (mg QE/g FruJu extract).
Determination of total polysaccharide content: The phenol-sulfuric acid method was used. Briefly, 100 μl of the FruJu was added to 100 μl of phenol at 5% and 500 μl of sulfuric acid (H2SO4) al 95 %, the mixture was incubated at RT for 15 min. Optical Density (OD) was measured at 490 nm in spectrophotometer (BioTeck, Epoch microplate) and distilled water plus H2SO4 was used as target. The results were expressed as mg of glucose equivalents per gram of FruJu (mg GE/g of FruJu). Glucose was used as standard to elaborate the calibration curve [16].
Analysis of proximal composition: Values of reducing sugars were obtained through the 3,5-dinitrosalicylic acid method. Water activity and humidity content were determined according to methods by the Association of Official Agricultural Chemists (AOAC) 978.18 and 931.04, respectively. The pH was carried out according with the AOAC 981.12 in 2 g of homogenized in a pH-meter, Metrohm model 744. To measure soluble solids, the AOAC 932.12 method was followed and the reading was performed in digital refractometer, Pocket PAL® 88S (Japan). Total fat was determined according with the 920.39C procedure described by the AOAC (1990), using a Soxhlet-type extractor by Tecator. Total dietary fiber content was determined by using the AOAC 958.29 method [17].
Analysis of antioxidant activity: The antioxidant capacity of the P. edulis extract was analyzed through the 1,1-Diphenyl-2- Picrylhydrazyl (DPPH) method; this method is based on quantifying the discoloration of this radical due to the interaction with a substance that can donate a hydrogen atom or transfer an electron. The DPPH is a stable free radical due to the delocalization of an unpaired electron. This delocalization also gives it a violet coloration, which absorbs spectrophotometrically in methanol at 517 nm. Adding antioxidants to the DPPH• radical produces its reduction; thus, the degree of discoloration is determined as percent inhibition of the DPPH• radical in function of the concentration, and is calculated in relation with the Trolox reactivity as standard of reference. The method was carried out according to the protocol by Gil et al., The sample of analysis consisted in 1 ml of FruJu and 99 ml of the DPPH dissolution (20 mg/l). The same amount of DPPH and 1 ml of the water solvent was used as reagent reference. A target was obtained by replacing the chromophore by methanol. Absorbance was analyzed at 517 nm in spectrophotometer (BioTeck, Epoch microplate), after 30 min of reaction at room temperature and in darkness. The results were expressed in Trolox Equivalent micromoles per gram of sample (μmol TE/g).
Cell culture: The cell lines used, SW480 and SW620, were obtained from the European Collection of Animal Cell Culture (ECACC, Salisbury, UK). Briefly, the cell lines were maintained and propagated in 75 cm2 Falcon culture flasks in Dulbecco's Modified Eagle's (DMEM) medium (Gibco) with 25 mM of glucose and 2 mM of L-glutamine, supplemented with 10% Bovine Fetal Serum (BFS)(Gibco), 100 U/ml penicillin, 100 μg/ml streptomycin (Gibco) and 1% non-essential amino acids (Gibco) taken as Maintenance Medium (MM). For the treatments with FruJu, the medium was modified, thus: the bovine fetal serum was diminished to 3% and supplemented with 10 μg/ml of Insulin, 5 μg/ml of Transferrin and 5 ng/ml of Selenium (ITS) and called Treatment Medium (TM). All the cell cultures were incubated at 37°C in 5% CO2 [18].
Cell growth: The Sulforhodamine B (SRB) method was used to study cell growth. Said method is based on the bond of the dye to the proteins of the cells that have bonded to the culture plates. Briefly 5,000 cells were seeded per well in 96-well plates at 37°C and 5% CO2. Their growth was monitored for 72 h, performing fixations every 6 h, fixation was performed for 1 h with 100 μl at 50% (v/v) of Trichloroacetic acid (TCA) at 4°C. The plate was washed with water to eliminate the TCA and was left to dry during the night; the cells were stained during 30 min with 100 μl of SRB solution (0.4%). The unbound dye was rinsed with acetic acid at 1% v/v and then the plate was left to dry during the night. Lastly, 200 μl of Tris 10 mM (pH 10.5) solution was added to each well and the plate was incubated with stirring for 30 min at 55 rpm. The OD value was measured at 490 nm in a microplate reader (BioTeck, Epoch microplate) [19].
Determination of the antiproliferative effect of the P. edulis FruJu: The assay was performed according with that established by Ramírez et al., 2019 with some modifications: briefly: 15,000 cells/well were cultivated from each cell line in 96-well plates, the final volume of treatment medium with the FruJu concentrations studied was 200 μl. The cells were incubated for 24 and 48 h. The medium was replaced with FruJu every 48 h. After the exposure period at the different juice concentrations (39.6, 52.8, 66.0, 132.0 μg/ml), the cultures were stopped by adding 100 μl of cold TCA (50% v/v) (MERCK) at 4°C during 1 h, the acid was washed and 100 μl of SRB (0.4% p/v diluted in acetic acid 1%) were added for 30 min at room temperature (RT), and then washed with acetic acid (1% v/v in distilled water). The plates were left to dry at RT during 24 h. To read the absorbance, the SRB bonded to the proteins was solubilized by adding 100 μl of Tris-HCl buffer (10 mM pH 10.5) to each well and stirred in orbital manner during 20 min. The optical density was read at 490 nm in a microplate reader (BioTeck Epoch microplate). The viability percentage was calculated by using the following equation:

Extraction of intracellular lipids: The Folch method was used with modifications. Briefly, 1,000,000 cells were seeded in polystyrene Petri dishes (60 × 15 mm in size) and were cultivated in DMEM medium with BFS at 3% during 24 h. When the cells reached 80% confluence, the culture medium was removed and 1 ml of lysis RIPA buffer was added (Hepes, NaCl, Triton and SDS, pH 7.6). The lysate was divided into three portions: 400 μl to determine cholesterol, 400 μl for triglycerides, and 200 μl to quantify proteins. An amount of 500 μl of chloroform and methanol solution (2:1) was added to the 400 μl of lysate; the mixture was left under stirring for 2 min, then 1,000 μl of NaCl at 0.09% were added and stirred for 1 min in vortex; thereafter adding 2000 μl of chloroform and centrifuged at 3,000 rpm during 2 min and the chloroform phase was removed to a new tube (this phase had the extracted lipids). Thereafter, the chloroform was evaporated at RT during 24 h; cholesterol and triglycerides were determined separately in the resulting residue with the Human Liquicolor® commercial kits, following manufacturer’s instructions. Lipid concentration was normalized with the protein concentration from each sample. Proteins were quantified through the Bicinchoninic Acid (BCA) method, using serum albumin for the calibration curve [20].
Statistical analysis: The results are expressed as the mean ± Standard Error (SE) or percentage. The data were analyzed by using one-way Analysis Of Variance (ANOVA) and complemented with the Bonferroni or Tukey-Kramer tests. To compare the groups, GraphPad Prism software V8 was used. Values of p<0.05 were considered statistically significant.
Results
Phytochemical and antioxidant characterization of the P. edulis FruJu: The phytochemical analysis of the P. edulis FruJu confirms the presence of various families of secondary metabolites of biological and pharmacological interest; among these, the study found phenols, anthocyanins, flavonoids, alkaloids and tannins (Table 1), with flavonoids having the greatest presence in the juice. Regarding the physicochemical parameters, this juice has acidic pH and high value of degrees Brix, attributed to the content of sugars present in it. The data also show that the juice has greater capacity of interacting with free radicals (DPPH) and lower reducing capacity (Table 1).
| Phytochemicals | Concentration or units |
|---|---|
| Total phenols | 75 ± 18.0 (mg GAE/g) |
| Total flavonoids | 109.9 ± 15.5 (mg CE/g) |
| Total Anthocyanins | 92.3 ± 4.3 (mg C-3-G/g) |
| Total tannins | 92.3 ± 4.3 (mg TAE/g) |
| Total alkaloids | 25.3 ± 2.0 (mg QE/g) |
| Total polysaccharides | 784.1± 18.8(mg EG/g) |
| Analysis of proximal composition | |
| Reducing sugars | 25.3 ± 2.0 mg/l |
| Water activity | 0.46 |
| % Humidity | 15.7 |
| pH | 3.06 |
| Degrees (°Brix) | 12 |
| % Total fat | 1.6 |
| % Fiber | 1.1 |
| % Solubility in water | 19 |
| Antioxidant activity | |
| DPPH | 785.3 ± 80.8 (µmol TE/g) |
g=gram of lyophilized extract; GAE=Gallic Acid Equivalent; C-3-G=Cyanidin, 3-Glucoside; QE=Quinine Equivalents; CE= Catechin Equivalent; TAE=Tannic Acid Equivalents. TE=Equivalent.
Table 1: Phytochemical characterization, analysis of proximal and antioxidant composition of the P. edulis fruit juice
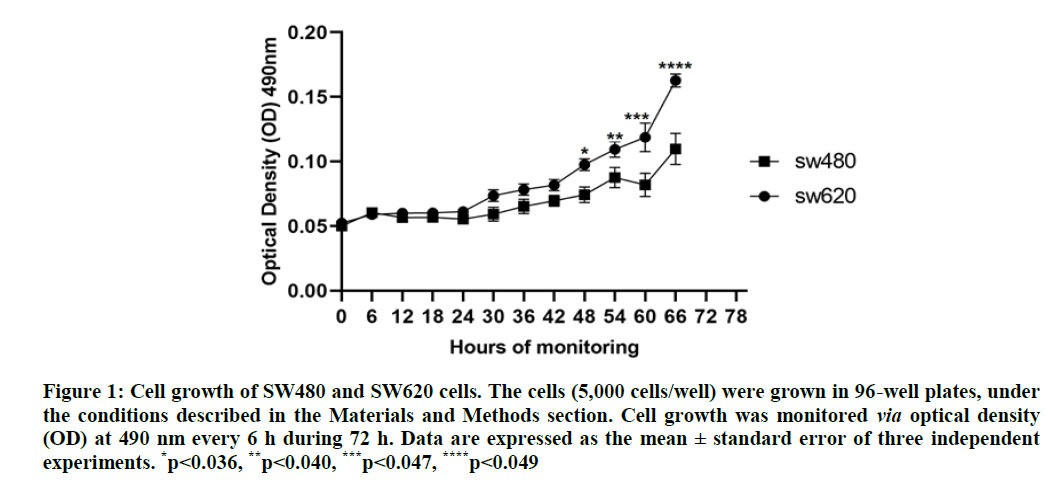
Cell growth analysis: Figure 1 shows the growth of the SW480 and SW620 cells. Under the culture conditions described in the materials and methods section, cell growth in both lines was similar during the first 24 h, as of which the growth of the SW620 cells was greater than in the SW480 cells with significant differences in each point evaluated. Both lines reach their exponential phase after 60 h, with the growth of the SW620 cells being 1.6 times greater compared with the SW480 cells (p=0.049).
Figure 1: Cell growth of SW480 and SW620 cells. The cells (5,000 cells/well) were grown in 96-well plates, under the conditions described in the Materials and Methods section. Cell growth was monitored via optical density (OD) at 490 nm every 6 h during 72 h. Data are expressed as the mean ± standard error of three independent experiments. *p<0.036, **p<0.040, ***p<0.047, ****p<0.049
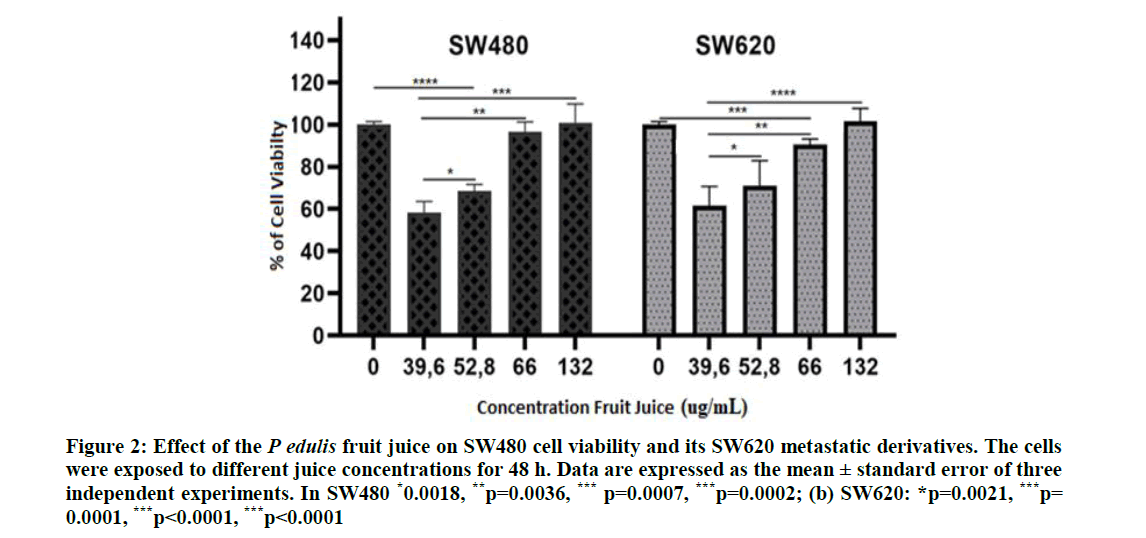
Effect of the FruJu on cell viability: Figure 2 shows the viability of both cell lines; growth of the SW480 cells diminished at FruJu concentrations of 39.6 and 52.8 μg/ml; although no differences were found between these two concentrations, differences were found with respect to the cells no treated with the juice (control group), a similar effect of the juice was observed in the SW620 cells. In addition, at concentrations ≥ 66.0 μg/ml, cell viability of both cell lines increased, showing inverse behavior to the known hormetic behavior in which there is a biphasic response to the stimulus of a natural agent.
Figure 2: Effect of the P edulis fruit juice on SW480 cell viability and its SW620 metastatic derivatives. The cells were exposed to different juice concentrations for 48 h. Data are expressed as the mean ± standard error of three independent experiments. In SW480 *0.0018, **p=0.0036, *** p=0.0007, ***p=0.0002; (b) SW620: *p=0.0021, ***p= 0.0001, ***p<0.0001, ***p<0.0001
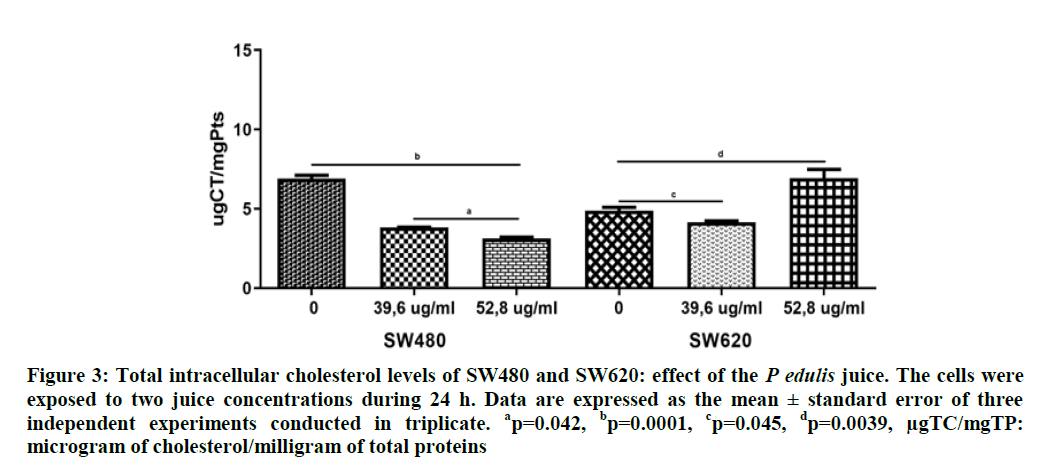
Effect of P. edulis FruJu on the intracellular cholesterol and triglyceride concentration: Figure 3 shows the intracellular concentration of Total Cholesterol total (TC) in SW480 and SW620 cells. Data indicate higher content of TC (6.9 ug/ml) in the SW480 cells compared with the SW620 (4.9 ug/ml) with significant differences (p=0.0025). The P. edulis FruJu at concentrations of 39.6 and 52.8 μg/ml diminished significantly intracellular cholesterol levels in the SW480 cells, 3.8 and 3.1 ug/ml, respectively (p=0.0001), while in the SW620 cells this decrease was only at the concentration of 39.6 μg/ml, (4.2 ug/ml) (p=0.045), with a recovery of cholesterol levels above the control values at 52.8 ug/ml.
Figure 3: Total intracellular cholesterol levels of SW480 and SW620: effect of the P edulis juice. The cells were exposed to two juice concentrations during 24 h. Data are expressed as the mean ± standard error of three independent experiments conducted in triplicate. ap=0.042, bp=0.0001, cp=0.045, dp=0.0039, µgTC/mgTP: microgram of cholesterol/milligram of total proteins
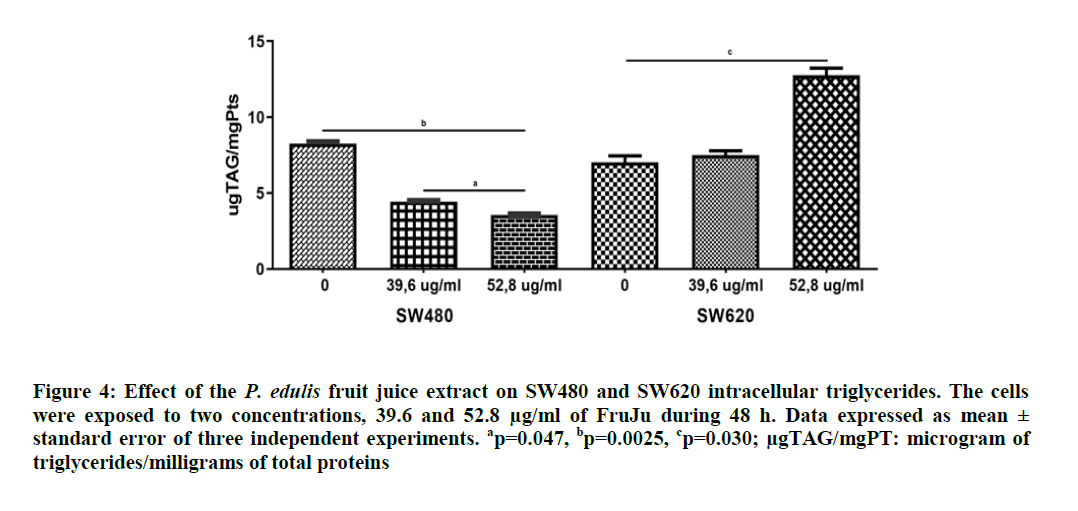
For the intracellular triglycerides, a similar situation was found, which evidenced that the SW480 cells have higher triglyceride content (8.8 ug/ml) than the SW620 cells (7.0 ug/ml) with significant differences (p=0.0037). The P. edulis FruJu managed to decrease triglycerides in SW480 at both study concentrations, 39.6 and 52.8 μg/ml by 4.4 and 3.6 ug/ml, respectively (p=0.007), but in SW620, the triglycerides increased at both concentrations (7.5 and 12.8 ug/ml, respectively), with significant differences the latter value (p=0.030), (Figure 4).
Figure 4: Effect of the P. edulis fruit juice extract on SW480 and SW620 intracellular triglycerides. The cells were exposed to two concentrations, 39.6 and 52.8 µg/ml of FruJu during 48 h. Data expressed as mean ± standard error of three independent experiments. ap=0.047, bp=0.0025, cp=0.030; µgTAG/mgPT: microgram of triglycerides/milligrams of total proteins
Discussion
Intracellular lipids are indispensable for tumor cell survival and migration and are used by said tumors to generate membranes, obtain energy and in signaling pathways hence, the study of lipid metabolism is of great interest in the search for therapeutic targets to fight cancer.
Medicinal plants constitute an undeniable source of bioactive substances used in the fight against cancer and, from them; various medications against this disease have been designed or established.
This work studied the FruJu from P. edulis, a plant from the Passifloraceae family for whose extracts different biological activities have been demonstrated, like its antiproliferative, antihypertensive, and antioxidant capacity but the mechanisms of these actions have not been fully described, nor which of its compounds may be attributed.
The phytochemical analysis of the P. edulis FruJu, described in this work, confirms the existence of various families of secondary metabolites of biological and pharmacological interest, among which were found anthocyanins, flavonoids, and alkaloids, indicating that the FruJu contains compounds to which the biological effects could be attributed as observed in the SW480 and SW620 cells in this study; besides, the FruJu used has acid pH and evidence shows that cancer cells acidify their environment and thereby, alkalinize intracellularly to proliferate; presence of an already acid culture medium or rupture of the proton gradient by the FruJu’s pH could limit internal alkalinizing and avoid proliferation of the cells studied.
Furthermore and synergizing with the effect of the FruJu’s pH on the cell viability, the antioxidant properties of the Juice measured by DPPH could be considered. Various studies indicate that antioxidants prevent and treat several types of malignant tumors, thus, antioxidants of polyphenol, anthocyanin, flavonoid, and tannin type, extracted from diverse plants, suppress in vitro proliferation of cells from gastric, colon, and lung cancer, among others which could explain the FruJu’s antiproliferative effect at low concentrations described in this work.
However, presence of reducing sugars and polysaccharides, related with the degrees Brix described for the FruJu would be playing a favorable role of proliferation that could explain the reverse hormetic effect observed in the study, in that greater concentration of the juice favors the viability of both cell lines, while low concentrations do not, given that studies have demonstrated for decades that the glucose metabolism plays a major role in oncogenesis.
In addition, the SW480 and SW620 cell lines used in this research show a different growth rate under the same culture conditions, especially during their exponential phase, a characteristic demonstrated by other authors. This growth rate difference may be due not only to their origin (SW480 primary tumor and SW620 metastatic cells), but to the expression of chromosome markers, factors and proteins that reflect different growth in distinct culture media (Thuringer).
Regarding cell viability, the FruJu’s concentration of 39.6 μg/ml obtained a comparable decrease (45.6% and 45.1%) in viability for SW480 and SW620, respectively, compared with their untreated counterparts. Nevertheless, at concentrations of the FruJu ≥ 52.6 μg/ml, increased cell viability was observed. A possible explanation for this result may be that the juice at these concentrations behaves as an anti-hormetic agent, a phenomenon contrary to the known hormetic behaviour (benefits are seen at low doses and harmful effects are seen at high doses). In this work, the antiproliferative effect (harmful) was obtained at low doses. Abundant evidence indicates that some compounds or natural extracts have an inverse effect or opposite to the hormetic, for example, Dang and Lowik (36) reported that the daidzein isoflavone showed biphasic effect on osteogenesis, but did so opposite/inversely to the hormetic effect; specifically, daidzein stimulated osteogenesis at concentrations from 1-20 μM), but at concentrations ≥ 30 μM, it inhibited osteogenesis.
An explanation to this effect may be as mentioned for the carbohydrate content present in the juice (high glucose and fructose content), both sugars are incorporated to the cell via transporters and stored or metabolized to provide energy for vital cell processes.
This phenomenon of differential growth against carbohydrates in non-tumor cell lines has been observed by authors, like Peres et al., who showed that at high glucose concentrations (30 mM), kidney cells in culture increased in number.
In this work, this growth behavior of the cell lines with respect to the FruJu is likely due to, on one hand, to a differential expression of glucose transporters for each cell line, as described by several authors for other cells, but, on the other hand, at low concentrations of the FruJu, in the tumor cells studied, possibly there is greater influence from the antiproliferative mechanisms of the phytochemicals present (via antioxidants) than the mechanisms used by carbohydrates to stimulate growth.
With regard to the cholesterol and triglyceride intracellular levels determined in the SW480 and SW620 cell lines, the intracellular content of these metabolites in this in vitro model is reported for the first time, as well as the effect of the FruJu on these. The results obtained in this study reveal that the response of these lipids to the FruJu was differential in both cell lines; that is, while the SW480 cells decrease, total cholesterol and triacyl glyceride levels of these lipids increase after treatment with the P. edulis juice, in their metastatic derivatives under the same treatment conditions treatment with juice. These findings suggest metabolic adaptations of the metastatic cells to favor their proliferation and evasion of tumor growth suppression mechanisms; in addition, these cell lines have different duplication times, SW480 25 ± 1 h and SW620 cells 23 ±1 h.
Mammalian cells require cholesterol to proliferate; once cell division begins, the cells activate the mevalonate pathway (cholesterol synthesis) increasing expression of Hydroxymethyl-Glutaryl-CoA Reductase (HMGCoAR), a key enzyme in the synthesis and receptor process of the Low Density Lipoproteins (LDL), to ensure cholesterol supply. Cholesterol and its precursors are important in maintaining the fluidity of the cell membrane, protein coupling to it, functioning of channels and receptors among other functions, besides participating in signaling pathways of the cell cycle. Research show reduced cell proliferation when cholesterol biosynthesis is inhibited in absence of an external source.
Several phytochemicals and plant extracts have been identified with capacity to reduce cholesterol levels in complete organisms and in cell models. Our results show decreased cellular proliferation in SW480 and SW620 from 45.6% and 45.1%, respectively, at a concentration of 39.6 μg/ml and at the same concentration a reduction of cholesterol levels (44.6% and 14.8%, respectively). Although the cause-effect relationship between lipids and decreased cell viability was not studied, a hypothesis that emerges from these findings is that the concentration at 39.6 μg/ml of FruJu inhibits cholesterol synthesis and affects proliferation of the SW480 and SW620 cells, especially in the SW480 cells, where the reduction of the cholesterol levels is greater, but further studies are needed to know the mechanisms involved in this cholesterol reduction and its relation with the antiproliferative effect observed herein.
A similar situation takes place for triglycerides, which diminished significantly by 46.52% at the concentration of 39.6 μg/ml in SW480, a concentration at which the FruJu reaches the highest antiproliferative effect in this cell line, also suggesting that triglyceride availability could be implied in the FruJu’s antiproliferative mechanism.
For the SW620 cells, the effect is different; the FruJu does not decrease triglycerides, on the contrary, triglycerides increase when the cells are exposed to 39.6 μg/ml, concentration at which the FruJu has the highest antiproliferative effect; further, at greater concentrations of FruJu, the triglycerides increase notably with respect to the untreated cells (control) with significant differences.
In this regard, the literature has demonstrated that triglycerides are used as source of energy, of re-composition of the cell membrane and of metabolites for signaling pathways in tumor cells, this membrane reconstruction, according to several authors, lets them modulate tolerance to oxidative stress and cell survival; moreover, these changes in the lipids permit invasion and metastasis, characteristics of the SW620 cells. Various studies have revealed that, unlike the primary tumor cells, metastatic cells activate several metabolic pathways of lipids, for example, they can improve the uptake of Fatty Acids (FA) by increasing the CD36, an FA transporter, over express carnitine palmitoyl transferase 1A (CPT1A, key rate-limiting enzyme for fatty acid oxidation), increase de novo lipid biosynthesis through the ATP-Citrate Lyase (ACLY) (a rate controlling enzyme of the first step in lipid synthesis) and Fatty Acid Synthase Enzyme (FASN) (key enzyme in fatty acid synthesis), which also promotes capacity for migration and invasion of the cancer cells. These characteristics of the metastatic cells in the metabolism of lipids could justify the differences of the FruJu effect on these compounds on both cell lines used herein.
Our findings are supported by various studies that have shown not only alterations in the lipid-energy metabolism of tumors and cancerous cell lines in general, but differences in the altered metabolic pathways in each of them, providing lipid phenotypes characteristic of each tumor or line, evidencing that lipids can be potential biomarkers of different types of cancer and can be used to classify some cancer cells, as described by Wang et al., who found 22 potential lipid biomarkers, classified into two classes, phosphatidylcholines and glycosphingolipids.
Also, some studies have shown that variations in the content and type of intracellular or membrane lipids are related with the resistance or sensitivity of cell lines to treatments with synthetic drugs, plant extracts, or individual or combined phytochemicals.
Conclusion
This work demonstrates different cell growth and lipid content in each line, which could be related with the antiproliferative effect of the FruJu de P. edulis, which at 39.6 μg/ml reduces cholesterol and triglycerides in SW480, but only cholesterol in SW620 and increases triglycerides in this line, concentration at which its greatest antiproliferative effect is found in both lines.
References
- Aguillón J, Maldonado ME, Loango N, et al. Perspectivas en Nutrition Humana. 2013;15(1):13–25.
- Amin M, Haghi A, Rahmati M, et al. Cancer Letters. 2018;424:46-69.
- Armentano MF, Bisaccia F, Miglionico R, et al. BioMed Res Int. 2015;2015.
- Bao Y, Wang W, Zhou Z, et al. PLoS One. 2014;9(12): e114764.
- Benarba B and Pandiella A. Biomed Pharmacother. 2018;107(6):408–423.
- Broadfield LA, Pane AA, Talebi A, et al. Dev Cell. 2021;56(10):1363–1393.
- Butler LM, Perone Y, Dehairs J, et al. Adv Drug Deliv Rev. 2020;159:245-293.
- de Carvalho APA, Conte-Junior CA. Trends Food Sci Technol. 2021; 111:534–548.
- Corn KC, Windham MA, Rafat M, et al. Prog Lipid Res. 2020;80:101055.
- Chen Y and Li P. Sci Bull. 2016; 61(19):1473–1479.
- Dang ZC, Löwik CW. J Bone Miner Res. 2004;19(5):853–861.
- Duranton B, Holl V, Schneider Y, et al. Amino Acids. 2003;24:63–72.
- Fazel S, Hamidreza M, Rouhollah G, et al. J Appl Hortic. 2010;12(1):69–70.
- García-Cardona DM, Landázuri P, Restrepo Cortés B, et al. Rev Colomb de Cienc Pecu. 2021; 33:94–101.
- Gazola AC, Costa GM, Zucolotto SM, et al. Biomed Pharmacother. 2018; 100(43):388–393.
- Ge S, Zhang Q, Tian Y, et al. Clin Chim Acta. 2020; 510:291-297.
- Gil Garzón MA, Rojano BA, Guerrero CA, et al. Corporación Universitaria la Sallista. 2012.
- Wang T, Chen X, Luan C, et al. Analytica Chimica Acta. 2020; 1107:92–100.
- Wosch L, dos Santos KC, Imig DC, et al. Brazilian J Pharmacogon. 2017; 27(1):40–49.
- Sun Y, He L, Wang W, et al. J Ethnopharmacol. 2021; 268