Original Articles: 2022 Vol: 14 Issue: 10
Optimization of Immediate Release Newer Version of Aripiprazole 30 mg Tablet using Box-Behnken Design
Anwar Khan1*, Md Sabir Alam2, Md Faheem Khan3, Mohsin Ali Khan3, Rupa Gupta4
1 Department of Pharmaceutics, ERA College of Pharmacy, ERA University, Lucknow, India
2 Department of Pharmacy, SGT University, Gurugram, Haryana, India
3 ERA Medical College and Hospital, ERA University, Uttar Pradesh, India
4 Sushant College of Pharmacy, Sushant University, Haryana, India
- Corresponding Author:
- Anwar Khan
Department of Pharmaceutics, ERA College of Pharmacy, ERA University, Lucknow, India
Received: 04-Nov-2022, Manuscript No. JOCPR-22-79014; Editor assigned: 08-Nov-2022, PreQC No. JOCPR-22-79014 (PQ); Reviewed: 22-Nov-2022, QC No. JOCPR-22-79014; Revised: 29-Nov-2022, Manuscript No. JOCPR- 22-79014 (R); Published: 06-Dec-2022, DOI:10.37532/0975-7384.2022.14(10).019.
Abstract
The aim of the investigation is to design pharmaceutically equivalent and quality improved immediate release oral dosage form of aripiprazole 30 mg tablet which is indicated for treatment of schizophrenia and related psychotic disorders in adults and in adolescent’s aged 15-17 year of age. Objective was established using Qbd approach and was systematically optimized using DoE, Box-Behnken design considering the effect of various independent variables and its interaction effect if there & establishing its impact on CQA’s. Aripiprazole is BCS class IV drug having low solubility and permeability with poor flow property and the level of drug substance was also kept around 10% w/w of core tablet which is very low, hence direct compression is not a suitable choice for manufacturing method. To achieve better wettability leading to more dissolution, and reducing relative standard deviation preferred manufacturing process was wet granulation process. Mathematical modelling was performed using linear model and response surface analysis was done to understand the factor-response relationship. The optimised formulation F-11 was found of high quality following the CQA’s within the specified limit, % drug release was found to 91.25% and % RSD was found to be 2.45%. It was clearly observed that there is significant impact of all three CMA’s (disintegrant, binder, diluent) on the quality attributes of the product (% release and content uniformity). The design showed linear model which depicts that there is no significant interaction effect observed and design space was plotted for working feasibility
Keywords
Immediate release; BCS; Qbd; % Drug release CMA’s; Box-Behnken design; DoE; RSD
Introduction
Tablet is the most popular among all dosage forms existing currently because of its convenience of self-administration [1], compactness and easy manufacturing [2]; however, in many cases immediate onset of action is required than conventional therapy, Oral route of administration is the most versatile route for systemic effects due to its ease of ingestion [3,4], convenience of self-administration, patient compliance, pain, compactness and easy manufacturing and most importantly versatility [5-8]. These formulations do not require sterile or aseptic conditions and are therefore, less expensive to manufacture [5-9]. Patient compliance, high-precision dosing, and manufacturing efficiency make tablets the solid dosage form of choice [10-15]. Oral administration of conventional dosage forms normally dissolves in the stomach fluid or intestinal fluid and absorb from these regions of the GIT depends upon the physicochemical properties of the drug that’s why it is regarded as the safest rote of administration [16], as the criticality and severity of adverse effects are lesser determined [17]. For many decades various pharmaceutical dosage form such as tablets, capsules, suppositories, creams, ointments, liquids, aerosols, and injectables have been used for the delivery of drugs to the patients for the treatment of various diseases [18-20]. Even today conventional dosage forms are the primary pharmaceutical vehicles commonly seen in the prescription and over the counter drug market [21-24].
Aripiprazole is an anti-psychotic drug for treating psychoses [25]. Abilify is indicated for the treatment of schizophrenia and related psychotic disorders in adults and in adolescents aged 15 -17 years of age [26-30]. Aripiprazole is indicated for the treatment of moderate to severe manic episodes in Bipolar I Disorder and for the prevention of a new manic episode in adults who experienced predominantly manic episodes and whose manic episodes responded to Aripiprazole treatment [31-33]. Aripiprazole is indicated for the treatment up to 12 weeks of moderate to severe manic episodes in Bipolar I Disorder in adolescents aged 13 -17 years of age [34-36]. Aripiprazole doses ranging from 0.5 mg to 30 mg administered once a day to healthy subjects for 2 weeks produced a dose-dependent reduction in the binding of C-raclopride, a D2/D3 receptor ligand, to the caudate and putamen detected by positron emission tomography [37]. It is marketed under the trade name Abilify by Bristol-Myers Squibb Canada in Canada which is innovator of the product [38,39]. Like other anti-psychotic drugs, the mechanism of action of Aripiprazole is unknown. Moreover, like other anti-psychotics [40-42], it blocks several receptors on the nerves of the brain for several neurotransmitters (chemicals that nerves use to communicate with each other). It is thought that its beneficial effect is due to its effects on dopamine and serotonin receptors. Since the formulation of immediate release Oro tablet using BCS class IV molecule involves multiple factors, the idea of optimization of the majorly influential factors on the IR dosage form using Design of Experiments (DoE) has been performed in the present work [43-45]. Nowadays, DoE approach is used rigorously in different fields of science like in analytical chemistry and formulation development for the optimization purpose to develop design space for producing quality at first time and every time [46].
Materials and Methods
Materials
Aripiprazole was obtained as a free sample from Ind-Swift Laboratories Ltd, Chandigarh, India (ISLL). Microcrystalline cellulose was obtained from FMC biopolymer, Island, HPC-L was obtained from Nippon soda as a gift sample from Mumbai, Maharashtra. Lactose monohydrate and Croscarmellose sodium were obtained from signet excipient pvt, ltd, Mumbai, Maharashtra. Magnesium stearate was obtained as a free sample from Nitika Pharmaceutical Specialties Pvt. Ltd, Nagpur.
Formulation of Aripiprazole Tablet
• Geometrically mixed Aripiprazole and a part of Lactose Monohydrate (Granu1ac-200) through suitable sieve.
• Sift together, Croscarmellose sodium (Ac-di-sol), Microcrystalline cellulose (Vivapur 102) though suitablesieve and mix with step 1.
• Dry Mixing- The materials of step no 2 is loaded into rapid mixer granulator and dry mixing for 10 minutes.Binder Preparation.
• Sift Hydroxypropyl cellulose (HPC-L) through suitable sieve.
• Dissolve the sifted HPC-L in water. Filter the binder solution through 60 mesh.
• Granulation: Add the binder solution in dry mix of Rapid mixer granulator with the help of peristaltic pump.Granulate the dry mix.
• Drying: Dried the granules in Fluid Bed Processor, at inlet temperature 75+5°C until LOD (@ 100°C) NMT 2%was achieved.
• Sifting and Milling-Pass the dried granules through sieve of mesh no 30 (ASTM). Collect the retained granulesover sieve & pass them through l.0 mm screen using Multi mill.
• Extra granular materials sifting-Sift Colloidal silicon dioxide (Aerosil 200 Pharma), Croscarmellose sodium(Ac-di-sol) through 30# and magnesium stearate sifted through #60 mesh.
• Blending: Load the sifted and milled dried granules and Colloidal silicon dioxide (Aerosil 200 Pharma),Croscarmellose sodium (Ac-di-sol) in conta blender and blend for 10 min at 10 rpm.
• Lubrication-Load sifted magnesium stearate to the bended materials in conta blender and blend for 5 min at 10rpm.
• Compression: The granules were compressed into tablets (Average weight 315 mg), using Ova1 concave punchtarget hardness.
The systematic optimization of aripiprazole tablet was carried out using Box-Behnken Design (BBD) with the help of Design Expert® ver. 9.0 software (Stat-Ease Inc., Minneapolis, USA). Three most influential factors including disintegration concentration, binder concentration and diluent concentration were selected as independent variable for optimization at 3 levels low (-1) medium (0) and high (+1) because as the molecule is categorised to BCS IV where the solubility and permeability both are low, it become important to use super disintegrant in adequate concentration to achieve its peak plasma concentration as the dosage form design is immediate release. Total of 13 experiments were suggested by selected design as shown in Table 1. % Drug release and % RSD were analysed as dependent variable or responses. After putting the data in BBD, mathematical modelling was performed to analyse the results (Table 2).
| Std | Run | Factor 1 | Factor 2 | Factor 3 | Response 1 | Response 2 |
|---|---|---|---|---|---|---|
| A: Croscarmellose sodium | B: HPC-L | C: Microcrystalline cellulose | Dissolution | Content uniformity | ||
| % w/w | % w/w | % w/w | NLT 85% in 30 min | NMT 5% RSD | ||
| 11 | 1 | 2 | 1 | 37.5 | 88 | 4.9 |
| 10 | 2 | 2 | 4 | 12.5 | 81.73 | 3.27 |
| 3 | 3 | 0 | 4 | 25 | 77.47 | 2 |
| 4 | 4 | 4 | 4 | 25 | 85 | 2.95 |
| 5 | 5 | 0 | 2.5 | 12.5 | 75.11 | 4.7 |
| 6 | 6 | 4 | 2.5 | 12.5 | 93.21 | 6.3 |
| 7 | 7 | 0 | 2.5 | 37.5 | 67.44 | 3.09 |
| 2 | 8 | 4 | 1 | 25 | 92.27 | 4.3 |
| 12 | 9 | 2 | 4 | 37.5 | 78.4 | 2.9 |
| 1 | 10 | 0 | 1 | 25 | 79.98 | 4.5 |
| 13 | 11 | 2 | 2.5 | 25 | 91.25 | 2.45 |
| 9 | 12 | 2 | 1 | 12.5 | 93 | 7.5 |
| 8 | 13 | 4 | 2.5 | 37.5 | 88.49 | 2.97 |
Table 1: Design matrix showing trial runs performed for optimization of Aripiprazole 30 mg tablet-using Box-Behnken Design
| Aripiprazole 30 mg Tablet Optimization | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ingredients | % W/W Formulation | ||||||||||||
| F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 | F10 | F11 | F12 | F13 | |
| Intra-granular | |||||||||||||
| Aripiprazole | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 | 10.00 |
| Lactose monohydrate | 48.50 | 70.50 | 60.00 | 56.00 | 74.00 | 70.00 | 49.00 | 59.00 | 45.50 | 63.00 | 59.50 | 73.50 | 45.00 |
| Croscarmellose sodium | 2.00 | 2.00 | 0.00 | 4.00 | 0.00 | 4.00 | 0.00 | 4.00 | 2.00 | 0.00 | 2.00 | 2.00 | 4.00 |
| Binder | |||||||||||||
| HPC-L | 1.00 | 4.00 | 4.00 | 4.00 | 2.50 | 2.50 | 2.50 | 1.00 | 4.00 | 1.00 | 2.50 | 1.00 | 2.50 |
| Purified water | QS | QS | QS | QS | QS | QS | QS | QS | QS | QS | QS | QS | QS |
| Extra-granular | |||||||||||||
| Microcrystalline cellulose | 37.50 | 12.50 | 25.00 | 25.00 | 12.50 | 12.50 | 37.50 | 25.00 | 37.50 | 25.00 | 25.00 | 12.50 | 37.50 |
| Lubricant | |||||||||||||
| Magnesium stearate | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
Note: QS: Quantity Sufficient.
Table 2: Percent w/w bill of material of formulation F1-F13
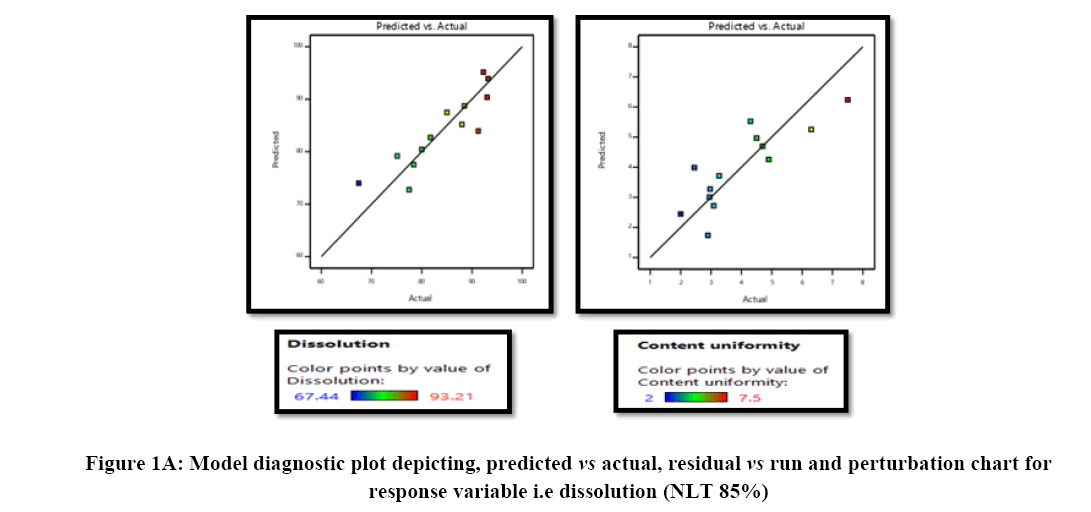
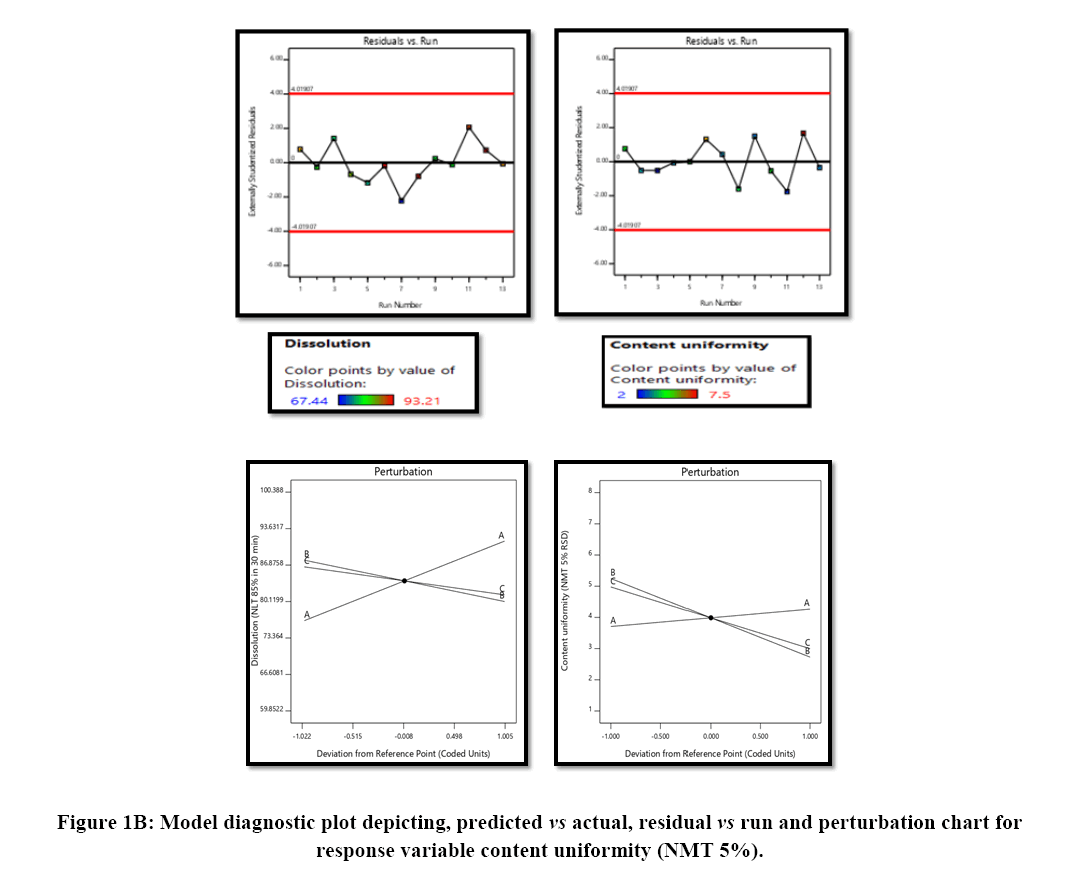
Linear model was selected and the data-fitting with the model was analysed by ANOVA along with other parameters like p-value, coefficient of correlation (r2), adjusted r2, predicted r2 and predicted residual sum of squares. Optimized concentrations required for development of aripiprazole tablet were identified by the numerical desirability function and graphical optimization technique (Figures 1A and 1B).
Result and Discussion
Selection of Dissolution Media Conditions and Quality Control Release Media
pH Solubility and Sink Condition- Aripiprazole API is insoluble in water. To understand the solubility of drug in the gastrointestinal pH range of 1-7.5, saturation solubility studies were carried out in various media using shake flask method at constant temperature (37˚C ± 2˚C). The solubility of Aripiprazole Crystalline Crystal B was examined in 0.l N Hydrochloric acid, 0.01 N HCl, 0.001 N Hydrochloric acid, Acetate buffer pH 4.5, Phosphate Buffer pH 6.0,Phosphate Buffer pH 6.8 and Phosphate Buffer (pH 7.5). The saturation concentrations found and based on this, sinkcondition, minimum volume and sink factors are presented in Table 3.
| Aripiprazole, Medium | |||||
|---|---|---|---|---|---|
| Media | Saturation Concentration (Solubility After 24 Hrs.) | Calculation for sink condition | Minimum volume (ml) | Calculation for sink factor | Sink Factor (900 mL) |
| 0.1 N HCI | 0.360 mg/ml | 1/0.360 × 30 × 3 | 250 | 0.360 × 1000/30 | 12 |
| 0.01 N HCl | 0.357 mg/ml | 1/0.357 × 30 × 3 | 252.1 | 0.357 × 1000/30 | 11.9 |
| 0.001 N HCl | 0.126 mg/ml | 1/ 0.126 × 30 × 3 | 714.29 | 0.126 × 1000/30 | 4.2 |
| Acetate buffer (pH 4.5) | 0.043 mg/ml | 1/0.043 × 30 × 3 | 2093.02 | 0.043 × 1000/30 | 1.43 |
| Phosphate buffer (pH 6.0) | 0.0012 mg/ml | 1/0.0012 × 30 × 3 | 75000 | 0.0012 × 1000/30 | 0.04 |
| Phosphate Buffer (pH 6.8) | 0.001 mg/ml | 1/0.0011 × 30 × 3 | 81818.18 | 0.0011 × 1000/30 | 0.04 |
| Phosphate Buffer (pH 7.5) | 0.00 mg/ml | 1/0.00 × 30 × 3 | Infinite | 0 × 1000/30 | 0 |
Table 3: Saturation concentration, sink condition, Minimum volume and Sink factor of Aripiprazole in various dissolution media.
As per this, sink condition (minimum volume) and sink factor (target: ≥ 3) are achieved in 0.l N HCl, 0.01 N HCI and 0.001 N HCl but not achieved in Acetate buffer pH 4.5, phosphate buffer pH 6.0, pH 6.8 and pH 7.5. It indicates that this API is sufficiently soluble in 0.l N HC1 dissolution media. As per CPMP guidelines on Bioavailability and Bioequivalence, CPMP/EWP/QWP/ 1401/98 Rev. 01, three different dissolution media {0.l N HCl (pH 1.2), Acetate Buffer 4.5 and Phosphate Buffer pH 6.8} have to be used for dissolution testing. Preliminary dissolution apparatus type, volume of media, RPM and dissolution time point were selected as per below selection criteria. And after that three multimedia dissolutions are selected in whole physiological pH range.
| Dissolution Conditions Method: Paddle Media: 0.IN HCI (900 ml) | ||||
|---|---|---|---|---|
| Time (Minutes) | Abilify 30 mg | |||
| RPM: 60 rpm | RPM: 50 rpm | |||
| Release | %RSD | Release | %RSD | |
| 10 | 85.43 | 4.98 | 80.2 | 9.32 |
| 15 | 93.41 | 2.62 | 84.46 | 7.93 |
| 20 | 96.55 | 1.19 | 89.87 | 6.46 |
| 30 | 98.62 | 1 | 92.66 | 3.45 |
| 45 | 99.3 | 1.01 | 94.68 | 2.5 |
| 60 | 99.49 | 0.98 | 95.47 | 2.55 |
Table 4: Comparative % Release of Innovator Abilify at Different RPM.
Aripiprazole in Various Dissolution Media
Dissolution apparatus: Dissolution apparatus type II is selected as generally acceptable apparatus Type II is selected for rapidly dissolving tablets and as per recommendations from USFDA OGD dissolution recommendations.
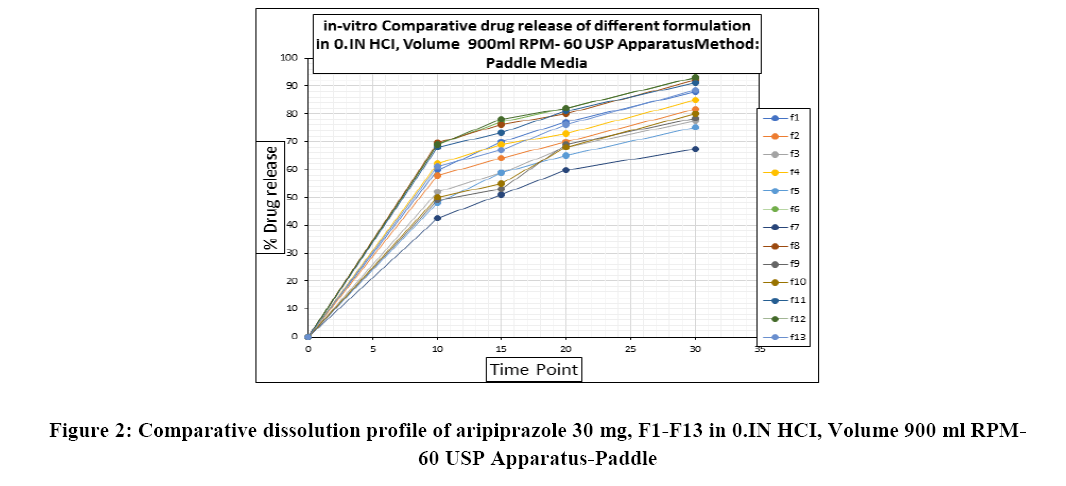
Volume of dissolution media: Dissolution media is selected 900 ml for the study as per standard volume and as per recommendations from USFDA OGD dissolution recommendations. RPM of Dissolution Apparatus-Paddle rotation speed was taken to access complete release of Abilify 30 mg innovator sample. The dissolution profile of Innovator Abilify 30 mg using paddles rotation speed of 60 rpm was acquired and compared to the data obtained with 50 rpm.
Release is incomplete in 60 minutes with 50 rpm. Based on this, 60 rpm is chosen for further dissolution. Moreover, the 60 rpm speed is in accordance with FDA proposed dissolution conditions.
Dissolution time: As target product is rapid dissolving tablets hence it was decided to keep 30 min dissolution time point.
Difference and Similarity Factor
Results obtained from the dissolution profile were fitted into equations (1) and (2) to determine the difference and similarity factors of the various batches compared to standard. Difference and similarity factors are model independent approach used to estimate the dissimilarity factor (f1) and similarity factor (f2) to compare the dissolution profile of optimized formulation (F5) with innovator product. The difference between the reference and test curve at each time point and is a measurement of the relative error between two curves. The FDA suggested that two dissolution profiles were declared similar if f2 value between 50-100 and f1 was 0-15.
f1= {(∑ t=ln |Rt-Tt|) / (∑t=ln Rt]) ×100 -- Equation (1)
f2=50• log {(1+ln∑t=ln (Rt−Tt) 2)-0.5×100} -- Equation (2)
Where, f1: Difference factor; f2: Similarity factor; n: time points; Rt: cumulative percentage dissolved at time t for the reference; Tt: cumulative percentage dissolved at time t for the test.
Content Uniformity
Results obtained from the dissolution profile were fitted into equations (1) and (2) to determine the difference and similarity factors of the various batches compared to standard. Difference and similarity factors are model independent approach used to estimate the dissimilarity factor (f1) and similarity factor (f2) to compare the dissolution profile of optimized formulation (F5) with innovator product. The difference between the reference and test curve at each time point and is a measurement of the relative error between two curves. The FDA suggested that two dissolution profiles were declared similar if f2 value between 50-100 and f1 was 0-15.
f1= {(∑t=ln |Rt-Tt|)/ (∑t=ln Rt)} ×100 -- Equation (1)
f2=50•log {(1+ln∑t=ln (Rt−Tt) 2) −0.5×100} -- Equation (2)
Where, f1: Difference factor; f2: Similarity factor; n: time points; Rt: cumulative percentage dissolved at time t for the reference; Tt: cumulative percentage dissolved at time t for the test.
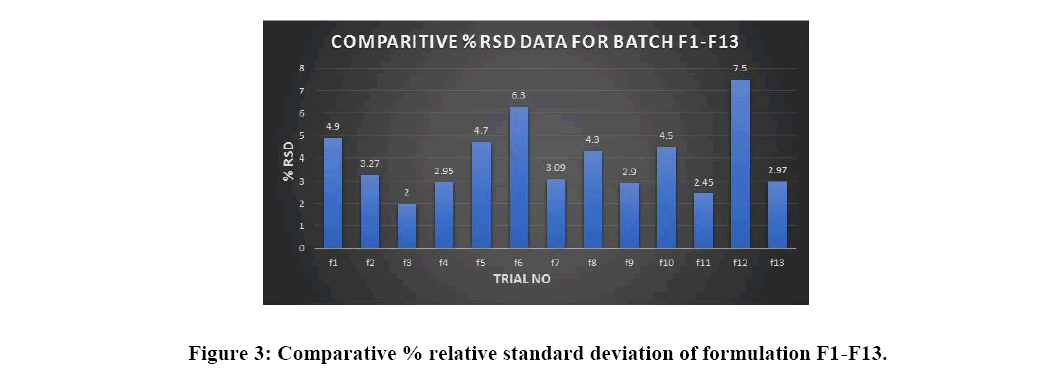
As the % of aripiprazole in aripiprazole tablet is less than 25% content uniformity analysis is mandatory rather than weight variation as the active ingredient is potent in nature. Mobile phase was prepared using Acetonitrile, methanol, Solution A, and glacial acetic acid in ratio (33:11:56:1) the internal standard solution of 0.33 mg/mL of USP Propylparaben RS in Mobile phase Standard, stock solution of 1 mg/mL of USP Aripiprazole Reference Standard in Mobile phase, Standard solution of 0.2 mg/mL of USP Aripiprazole Reference standard prepared- Transfer 10.0 mL of Standard stock solution and 10.0 mL of Internal standard solution to a 50 mL volumetric flask, and dilute with Mobile phase to volume. Sample solution: Nominally 0.2 mg/mL of aripiprazole from Tablets prepared as follows. Powder NLT 20 Tablets and transfer a suitable portion of the powder to an appropriate volumetric flask. Add 40% of the final flask volume of Mobile phase and 20% of the final flask volume of internal standard solution. Shake for 10 min, and dilute with Mobile phase to volume. Centrifuge, if necessary, and pass the supernatant through a suitable filter of NMT 0.5 µm pore size, discard the first 1 mL of filtrate, and use the subsequent filtrate (Figure 3).
Mode- HPLC was used, detecting wavelength of UV 254 nm, Column: 4.6 mm × 25 cm; 5 µm packing L1, Flow rate of 1 mL/min, Injection volume of 10 µL, run time of NLT 2 times the retention time of aripiprazole calculation of content uniformity was done using equation no 3.
CU= (peak response ratio of aripiprazole to the Sample solution)/(peak response ratio of aripiprazole to the Standard solution) × (concentration of RS in the Standard solution)/(concentration of aripiprazole in the Sample solution) × 100
Content uniformity = (RU/RS) × (CS/CU) × 100
Optimization of Aripiprazole 30 mg Tablet
The optimization of aripiprazole tablet was systematically optimized using BBD by Design Expert® ver. 9.0 software (Stat-Ease Inc., Minneapolis, USA). Dissolution (NLT 85%) and content uniformity (RSD NMT 5%) of tablets were optimized as responses. And the impact of disintegrant concentration, binder concentration and diluent concentration as independent variables (factors) at three different levels, viz., low (-1), medium (0) and high (+1) was established. A total of 13 experimental trials were suggested by BBD by Design Expert® ver. 9.0 software. The obtained data was subjected to fitting with the linear model and fitting analysis was performed using various
statistical parameters. Equation 1 and 2 were obtained as the equations generated after the data modelling, which indicates that there is no interaction effect and curvilinear effect for both the response variables analysed (dissolution and content uniformity). The parameters like coefficient of correlation were found good in the range between 0.78 (for dissolution) and 0.70 (for content uniformity), along with The Predicted R2 in reasonable agreement with the Adjusted R² i.e., the difference is less than 0.2, Adequate Precision ratio greater than 4 is desirable and achieve with linear model showing significant model for use. Hence, this model can be used to navigate the design space. Moreover, the model diagnostic plots for the responses are illustrated Figure 1 indicating good fitting of the data with the selected model.
Dissolution = +83.95 * A + 7.37 * B - 3.83 * C - 2.59 ………………………………Eq 1
Content uniformity = +3.99 * A + 0.2787 * B - 1.26 * C - 0.9888 …………………..Eq 2
By default, the high levels of the factors are coded as +1 and the low levels are coded as -1. The coded equation is useful for identifying the relative impact of the factors by comparing the factor coefficients.
Factor-response Relationship and Response Surface Methodology
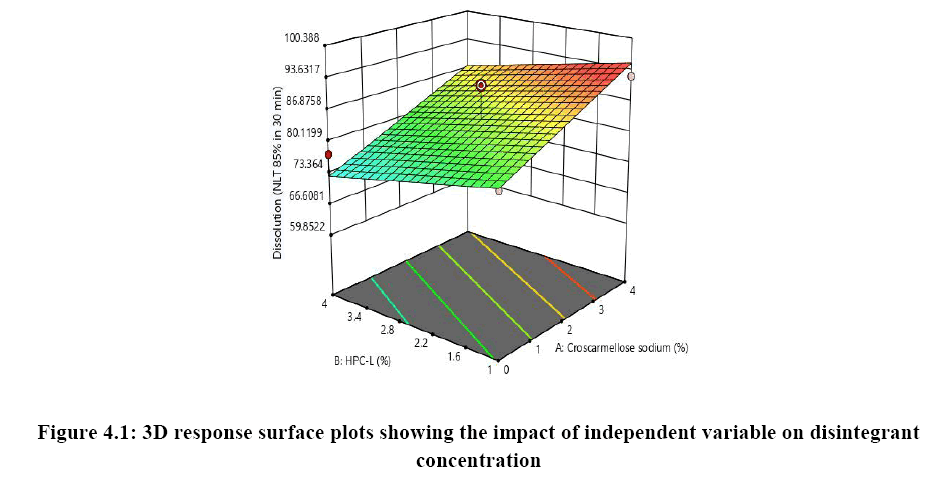
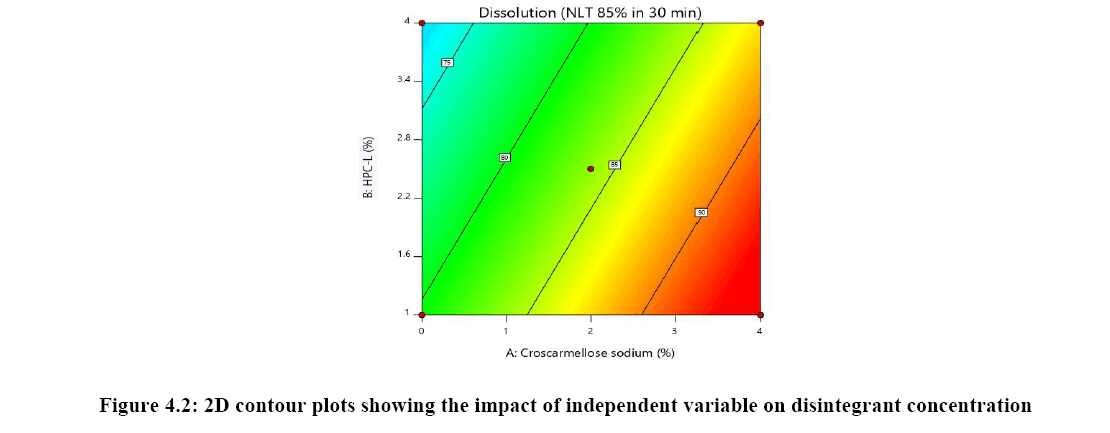
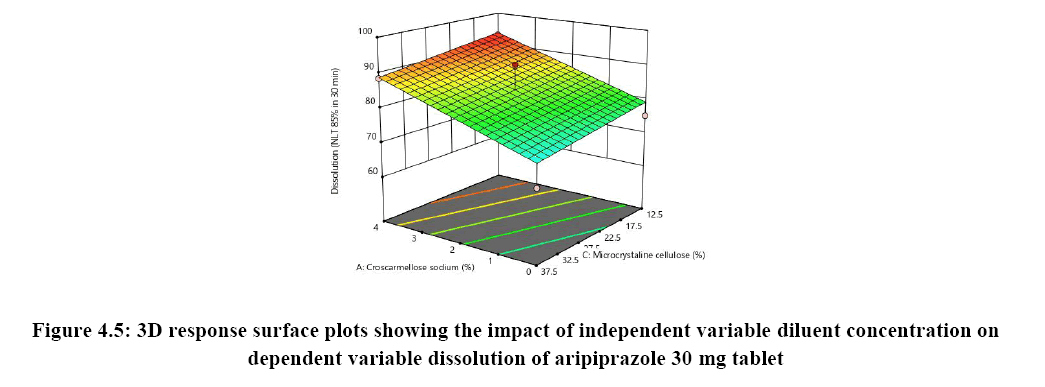
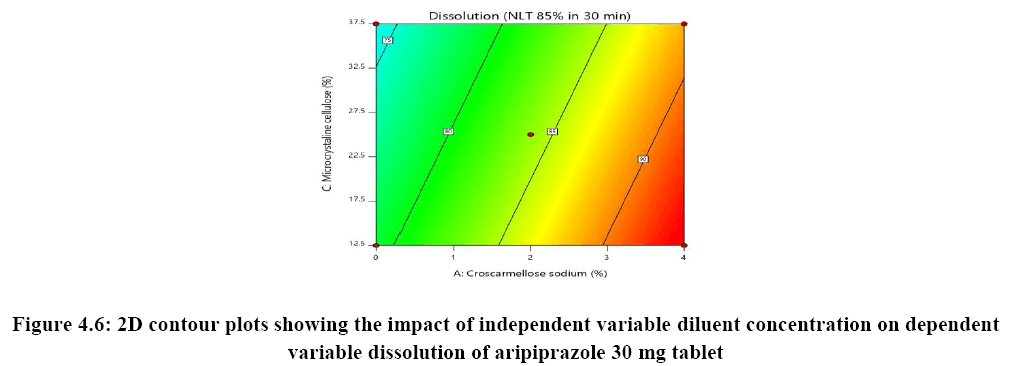
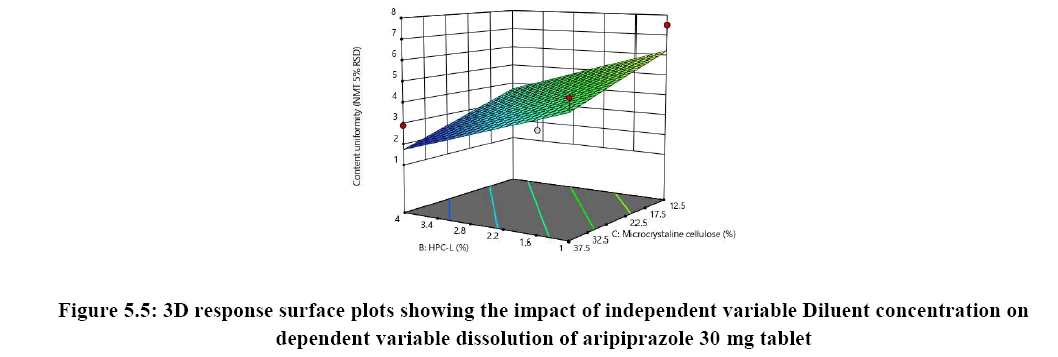
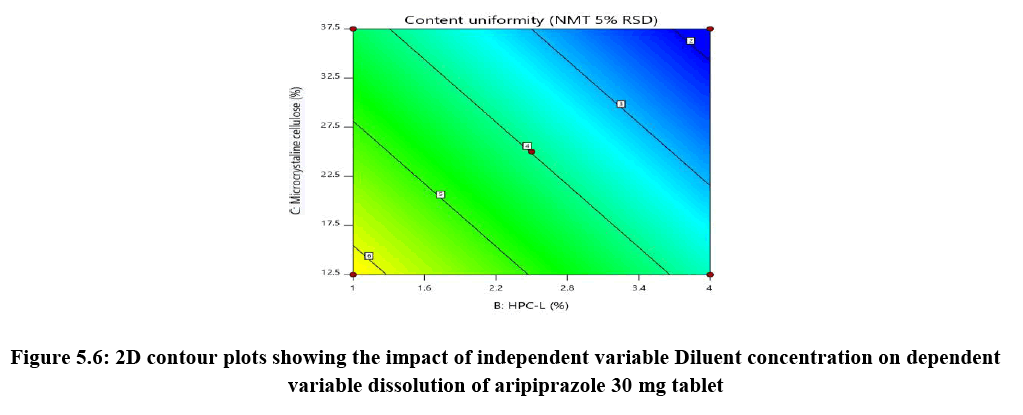
Response surface analysis was carried out using 3D response surface plots and 2D contour plots, which explained the absence of interactions among the independent variables and their influence(s) on the response variables. The response surface analysis plots for dissolution Figure 2. The relationship between concentration of HPC-L and concentration of croscarmellose is shown in Figure 4A. This indicated that there is no interaction effect as the plots are straight line in 3D response surface and has a significant impact on dissolution, where increase in the concentration of disintegrant and decrease in concentration of HPC-L increases the drug release of tablet. However, impact of concentration of croscarmellose is much more significant that the impact of HPC-L on % drug release as the p-value of croscarmellose is 0.0009 as compare to p-value of HPC-L 0.0329, in Figure 4B concentration of HPC-L have inversely proportional effect on % drug release as the concentration is reduced, release of the drug is enhanced but in combination with concentration of MCC it has very mild effect as the impact of mcc on % drug release is not significant p value for mcc is 0.1229 which is not significant very slight impact or noise effect can be observed for mcc on % drug release, same as in Figure 4C shows a sharp increase as the concentration of disintegrant is increase with a mild synergistic effect as the concentration of mcc is reduced.
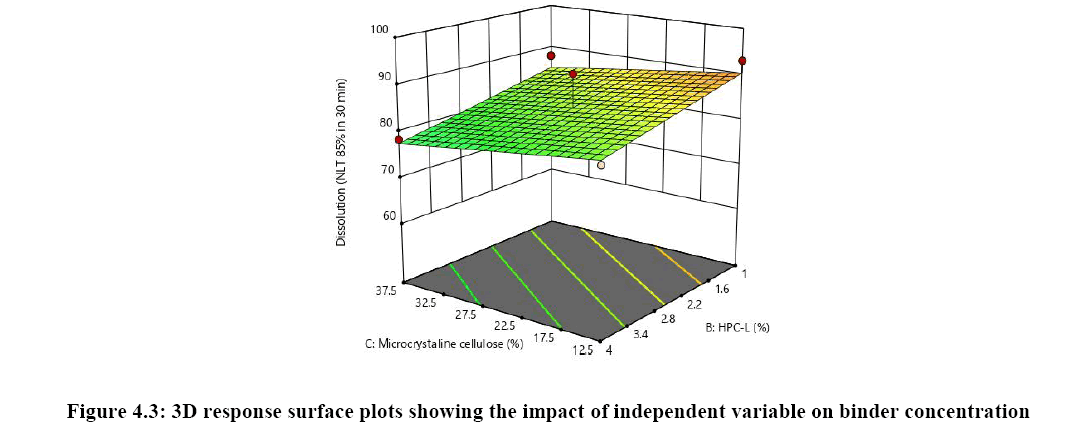
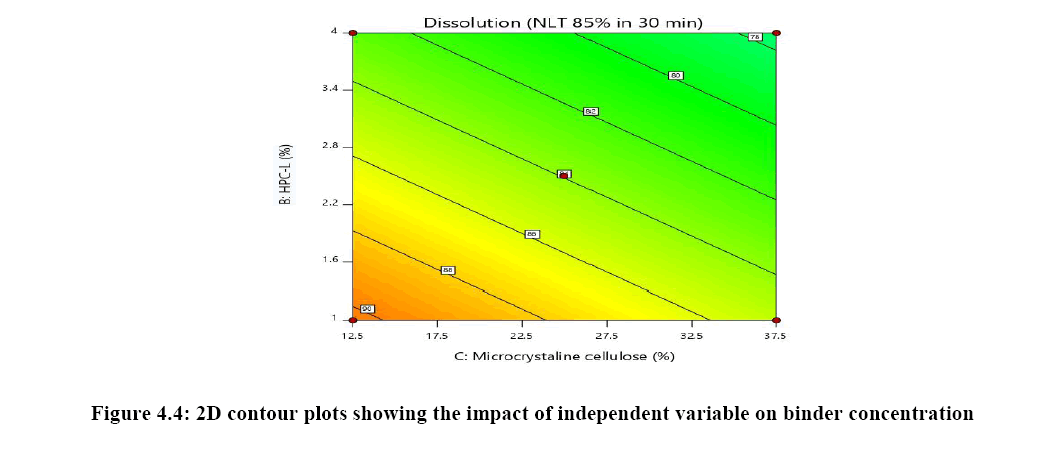
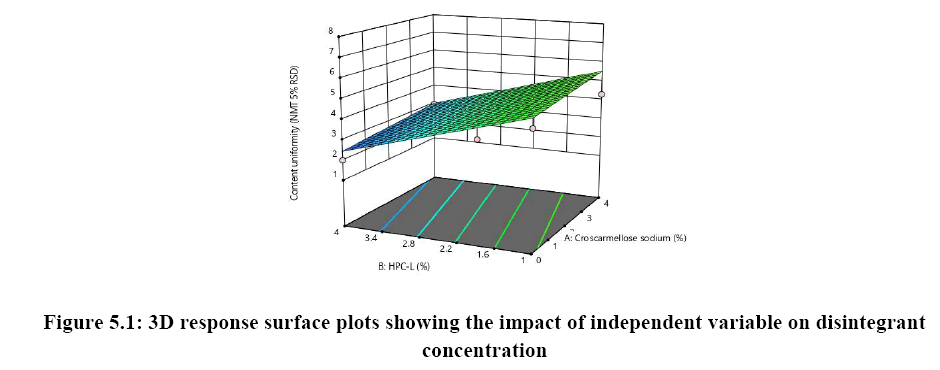
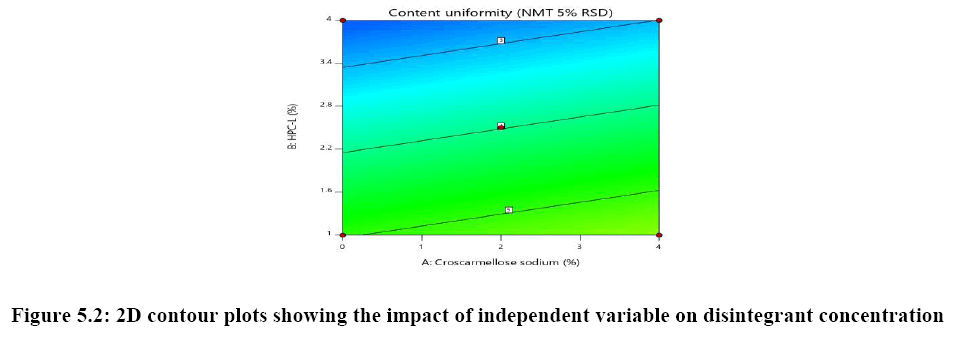
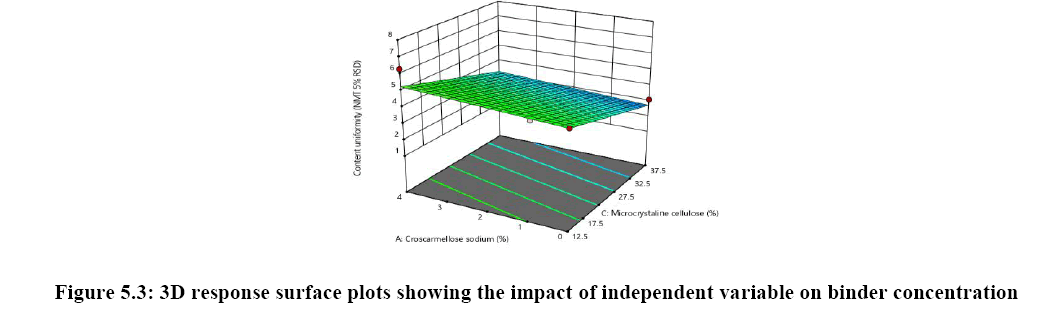
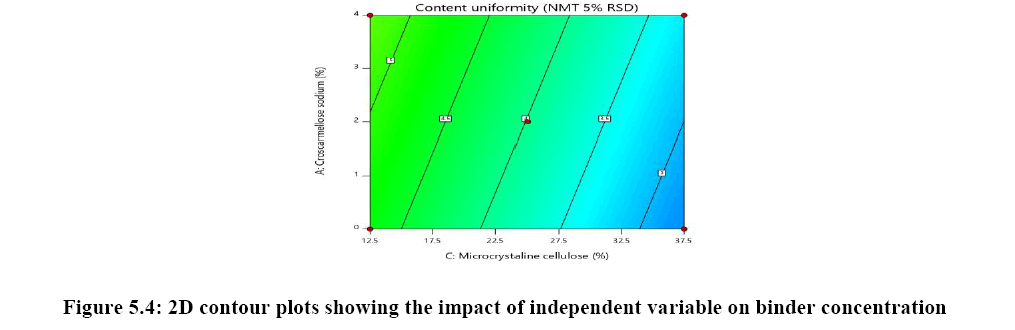
The relationship between independent variable as concentration of disintegrant, binder and diluent on dependent variable content uniformity is explained in Figure 5A where the of binder has significant impact on content uniformity as the p-value is 0.0064 as the concentration of binder is increased % RSD is reduced and uniformity of tablets are achieved whereas concentration of croscarmellose sodium has very mild or noise effect on response. There is no interaction effect observed as the response curve plotted is linear, whereas in both Figures 5B and 5C plot it was observed that major significant effect on response was observe because of concentration of HPC-L and concentration of microcrystalline cellulose. As the p-value for HPC-L is .0064 and p-value for mcc is 0.0217 both are having significant impact on response. As the concentration of mcc is increased %RSD is reduced.
Prediction of Optimized Formulation
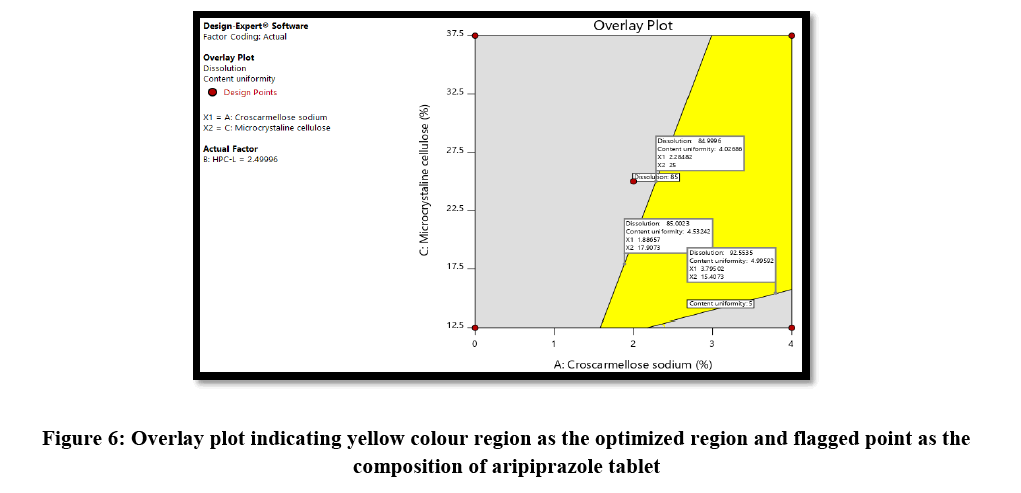
The optimized formulation of aripiprazole 30 mg tablet identified by numerical optimization with desirability function value closer to 1. The target goals for each of the response variable were provided, which included enhancing of % drug release NLT 85% in 30 min and reducing % RSD NMT 5%. The overlay plot indicated the yellow colour region as the optimized region that working within this region will be considered to have response within specified limit along with the flagged point representing concentration of croscarmellose, concentration of HPC-L and finally concentration of microcrystalline cellulose, this also provide that the response specification of NLT 85% dissolution and NMT 5% RSD for content uniformity would be achieved when working in yellow region of design space (Figure 6).
Conclusion
In the current study, optimised stable aripiprazole 30 mg immediate release tablet were formulated by using wet granulation technique using disintegrant in intra-granular part and diluent microcrystalline cellulose in extra granular part. The immediate release aripiprazole tablet was systematically optimized using Box-Behnken design considering the effect of various independent variables (factors) like concentration of croscarmellose (disintegrant), concentration of binder (HPC-L) and concentration of diluent (microcrystalline cellulose) temperature on the responses like dissolution (% drug release) and content uniformity (%RSD) of immediate release tablet were optimized. Formulation no 1,4,6,8,11,12, and 13 are following 1st criteria of dissolution should be more than 85% but when % RSD is compared of the batches only f-4, f-8, f-11 and f-13 are within specification limit of % RSD, f-11 is well suited optimized formulation showing release of 91.25% and % RSD of 2.45%. Further f-11 was moved forward for validation. The design showed linear model which depicts that there is no significant interaction effect observed and design space was plotted for working feasibility.
Conflict of Interest
The authors have no conflicts of interest regarding this investigation.
Acknoledgments
Authors are thankful to Ind-Swift Laboratories Limited in providing Aripiprazole. FMC biopolymer, Island for providing Microcrystalline cellulose, Signet excipient pvt, ltd, Mumbai, India for providing lactose monohydrate and croscarmellose, Nitika pharmaceutical specialities pvt. ltd. Nagpur, India, for providing magnesium stearate, Nippon soda as a gift sample from Mumbai, Maharashtra providing HPC-L.
References
- Hasnain MS, Rao S, Singh MK, et al. Analyst. 2013; 138:1581-1588.
- Hasnain MS, Rao S, Singh MK, et al. J Pharm Bioallied Sci. 2013; 5(1):74-79.
- Malakar J, Das K, Nayak AK. Polim Med. 2014; 44(4):221-230.
- Khan A, Alam MS, Hasan R, et al. Neuro Quantol. 2022; 20(10):10553-10570.
- Khan A, Hasan R, Gupta R, et al. WJPPS. 2022; 11(9):910-919.
- Jain K. 1st edition. CBS Publisher. 2004:82-90.
- Nair AB, Gupta R, Kumaria R, et al. J Basic Clin Pharm. 2010; 1(4):215-221.
- Sumit C, Sibaji S, Kumar DS. J Chem Tech Res. 2009;1(3):663-666.
- Wolf J, Janssen F, Lublin H, et al. Curr Med Res Opin. 2007;23(10):2313-2323.
- Masanori KU, Toshiko KO, Maune H, et al. Drug Metab Pharmacokinet. 2007;22(5):358-366.
- Buchanan RW, Freedman R, Javitt DC, et al. Schizophrenia Bulletin. 2007;33(5):1120-1130.
- Huang HC, Liu CH, Lan TH, et al. J Chromatogr B Analyt Technol Biomed Life Sci. 2007;856(1-2):57-61.
- Howland RH. J PsychosocNursMent Health Serv. 2007; 45(5):15-18.
- Obradovic M, Mrhar A and Kos M. Int J Clin Pract. 2007;61(12):1979-1988.
- Bond DJ, Pratoomsri W and Yatham LN. Acta Psychiatr Scand Suppl. 2007;(434):3-16.
- Yokoi F, Gründer G, Biziere K. Neuropsychopharmacology. 2002; 27(2):248-259.
- Sireesha P, Mahammed NL, Reddy KNK. AJPRD. 2014; 2(2): 125-133.
- Tokisato K, Fukunaga K, Tokunaga M, et al. Intern Med. 2015;54(23):3061-3064.
- Simon N, Azorin JM. Encephale. 2018; 44(6):558-564.
- Han M, Huang XF, Deng C. Int J Neuropsychopharmacol. 2009;12(7):941-952.
- De BA, Tomasetti C, Iasevoli F. CNS Drugs. 2015; 29(9):773-799.
- Urban AE, Cubała WJ. Psychiatr Pol. 2017; 51(6):1059-1077.
- Russo L, Di VA, Rizzo A. Curr Drug Saf. 2019;14(2):155-157.
- Swainston HT, Perry CM. Drugs. 2004; 64(15):1715-1736.
- Zak T, Chowhan T. 8th edition. Ansel’s Pharmaceutical Dosage Forms and Drug Delivery System’. 2002;3(7):31-40
- Sameer GL. Yu YY, Banga AK. Int J Pharm. 2009; 365(1-2):4-11.
- Baldessarini RJ, Tarazi FI.
- Keith S. Prog Neuropsychopharmacol Biol Psychiatry. 2006;30(6):996-1008.
- Keck PE, Calabrese JR, McIntyre RS, et al. J Clin Psychiatry. 2007;68(10):1480-1491.
- Rabin C, Liang Y, Ehrlichman RS, et al. Schizophr Res. 2008; 98(1-3):66-78.
- Hirsch LE, Pringsheim T. Cochrane Database Syst Rev. 2016 (6).
- Aggarwal A, Schrimpf L, Lauriello J. Clin Schizophr Relat Psychoses. 2018;11(4):221-223.
- Prommer E. Am J Hosp Palliat Care. 2017; 34(2):180-185.
- By the 2019 American Geriatrics Society Beers Criteria® Update Expert Panel. J Am Geriatr Soc. 2019; 67(4):674-694.
- Brunetti M, Tizio DL, Dezi S, et al. Eur Rev Med Pharmacol Sci. 2012;16(10):1346-1354.
- Tuplin EW, Holahan MR. Curr Neuropharmacol. 2017;15(8):1192-1207.
- Raedler LA. Am Health Drug Benefits. 2016; 9:40-43.
- Pondé MP, Freire AC. Case Rep Psychiatry. 2015; 2015:419746.
- Biederman J, Mick E, Spencer T, et al. CNS Spectr. 2007; 12(9):683-689.
- Sachs G, Sanchez R, Marcus R. et al. J Psychopharmacol. 2006;20(4):536-546.
- Nyilas M, Carson W, Forbes R. et al. Sch Res. 2008;98:48.
- D Souza, Sutar PS, Sutkar KP, et al. IJRAP. 2012; 3(4):597-603.
- Leite JV, Guimaraes FS, Moreira FA. Eur J Pharmacol. 2008; 578(2-3):222-227.
- Lee TWY, Robinson JR. In Remington: The Science and Practice of Pharmacy; Limmer. 2000; 1069-1070.
- Loyd V. Allen J, Nicholas G, et al. Lippincott Williams and Wilkins, Philadelphia. 2006.
- Saptarshi D, Mukul S. J Pharm Res. 2009; 2(11):1728-1729.