Original Articles: 2025 Vol: 17 Issue: 1
Levels and Potential Health Risks of Some Organic Contaminants in Locally Produced Alcoholic Beverages in Nigeria
Ekere NR*, Inainfe E, Okoye COB, Ihedioha JN
Department of Pure and Industrial Chemistry, Analytical/ Environmental Chemistry Laboratory, University of Nigeria, Nsukka, Nigeria
*Corresponding Author:
- Ekere NR
Department of Pure and Industrial Chemistry, Analytical/ Environmental Chemistry Laboratory, University of Nigeria, Nsukka, Nigeria
Received: 19-Nov-2023, Manuscript No. JOCPR-23-120524; Editor assigned: 21-Nov-2023, PreQC No. JOCPR-23-120524 (PQ); Reviewed: 05-Dec-2023, QC No. JOCPR-23-120524; Revised: 09-Jan-2025, Manuscript No. JOCPR-23-120524 (R); Published: 16-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).235.
Citation: Ekere NR, et al. 2025. Levels and Potential Health Risks of Some Organic Contaminants in Locally Produced Alcoholic Beverages in Nigeria. J. Chem. Pharm. Res., 17:142.
Copyright: © 2025 Ekere NR, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
The purpose of this study was to determine the concentration of organic contaminants present and its associated human health risk. Seven major contaminants methanol, propanol, acetaldehyde, benzene, benzaldehyde, ethyl acetate and ethyl carbamate were analyzed in different samples of our locally manufactured and exported alcoholic beverages in Nigeria. Analysis were carried out using Gas-Chromatography coupled with Flame Ionization Detector (GC-FID). The Estimated Daily In-take (EDI) and Target Hazard Quotient (THQ) were assessed in the beverages. A total of 81 samples of nine different brands (9 for each brand) of our locally manufactured alcoholic beverages were analyzed. The results showed mean concentration (mg/l) ranges of the organic contaminants (Samples A-I) as follows methanol=(0.021 to 2.761), propanol=(N.D to 0.926), ethyl acetate=(0.024 to 3.807), benzaldehyde=(N.D to 0.873), benzene=(N.D to 0.018), Acetaldehyde and ethyl carbamate were not detected in any of the samples analyzed.. Based on the results, most of these spirit drinks might not be good for consumption due to benzene content which is a known carcinogen as declared by IARC and also methanol which was found in all the samples in concentrations higher than the permissible limit set by NAFDAC except in sample I. The human health risk assessment carried out in this work showed the values of the target hazard quotient was found to range from 1.8 × 10-5–4.0 × 10-3 in methanol, 4.2 × 10-4 –7.7 × 10-4 in propanol, 5.4 × 10-5–7.0 × 10-3 in ethyl acetate, 4.7 × 10-6–1.5 × 10-2 in benzaldehyde, while the carcinogenic human health risk assessment showed the health risk index to be 7.8 × 10-3 for benzene. All the samples had THQË?1 for the organic contaminants which means they pose no significant risk.
Keywords
Alcoholic beverages, Methanol, Ethyl acetate, Benzene, Acetaldehyde, Propanol, Ethyl carbamate, Benzaldehyde, Gas chromatography
Introduction
The word beverage is derived from a Latin word "Bever" (which means relaxation or a reaction to work). It is used as a drink to quench thirst, introduce fluid to the body, nourish the body and stimulate or calm a person [1]. Alcoholic beverages can be defined as a beverage with a particular level of ethanol in it, standard definitions for alcoholic drinks differ among nations due to difference in the minimum alcohol concentration standards [2]. The initial purpose of spirits and liqueurs was as remedies and elixirs for the sick. The method of production dates back thousands of years, but the original purpose has long since been abandoned because spirits and alcohols are now mostly used for enjoyment purpose other than for their potential medical or healing benefits. The Middle ages was when spirits were first discovered and developed, Wine and beer were the most popular drinks at the time and it was well known that they were intoxicating [3].
Alcohol is any organic compound with one or more hydroxyl groups attached to the carbon atom. It is a liquid that is produced when sugars naturally ferment, it is colorless, volatile and flammable [4]. Alcoholic beverages include any drinkable liquid with 5% to 95% ethanol, which is their main physiologically active ingredient [5]. Grains, fruits and vegetables are required to ferment before it can produce alcoholic beverages, the process of fermentation is when yeast or bacteria react with the sugars in food and the by-products are ethanol and carbon dioxide.
In alcoholic beverages, the alcohol content is affected by how long it is left to ferment, during fermentation not all the sugar is converted to ethanol, instead other alcohols like methanol and propanol are produced. These kind of alcohols should not be consumed in a concentrated form La Villa J. Due to the actions of indigenous bacteria like Saccharomyces cerevisiae, some traditionally processed beverages, including palm wine, have a poor shelf life and are not bottled, Millions of people generally drink palm wine in West Africa, particularly in Nigeria. As the tapping duration lengthens, the sugar content decreases and the alcohol concentration rises [6]. Wine, spirits and beer make up the majority of alcoholic beverages, other alcoholic beverages including palm wine are sold in cans, bottles and sachets, also in nations where it is not customary, beer consumption has surged during the past few decades [7]. In Nigeria, some locally produced popular alcoholic beverages include; Bull gin, Chelsea dry gin, Calidon, Captain Jack, One-man Squad, Action Bitters, etc. are commonly referred to as hot drinks (Spirit) [8].
Wine and cider are made from fermented fruits, whereas beer and spirits are made from fermented cereals and barley, though spirits go through a distillation process that removes some of the water, resulting in a more concentrated alcohol. According to the World Health Organization (WHO), people from 15 years of age and above consume an average of 6.2 liters of pure alcohol per year, which equates to 13.5 g of pure alcohol per day [9].
Spirits account for 50.1% of total recorded alcohol consumption in the world, whereas beer accounting for the second largest proportion (34.8%) consumption. In addition, 8.0% of the total recorded alcohol is consumed in the form of wine and other alcoholic beverages (e.g., fortified wines, rice wine, or other fermented alcoholic beverages) represent 7.1% of the total alcohol consumption [9]. Chemical contaminants are the presence of chemical compounds where they should not be present, or at levels higher than what is considered safe, as this can have serious health consequences, either acute or long term [10]. Contamination routes is found in nearly every possible step in the production process, from the raw materials stage to the processing, during the storage process and at the last stage of packaging and sale.
Gin has an ABV that ranges from 35% to 55% and is a grain-based alcohol produced from juniper berries [11]. Whiskey is a spirit derived from fermented grains, it is sometimes referred to as a spirit beverage made from malted grain that has undergone diastase to become malted. The ABV varies between 40% to 50%. Rum is a spirit produced by distilling either fermented sugar cane juice, molasses, or a combination of the two. It contains 40% alcohol, which is the usual amount. Tequila is a Mexican distillate that gets its fermentable sugars from the agave plant. Tequila has an Alcohol by Volume (ABV) of roughly 40% [3]. Vodka is produced from ethanol of agricultural origin obtained by the yeast fermentation of potatoes and grains, then followed by distillation. Vodka must have a minimum ABV of 37.5%. To offer unique organoleptic qualities, such as a mellow flavor, some extra flavorings may be added Katarzyna P, et al. [12].
According to Ohimain EI, he reported that different cases of methanol poisoning have been reported in different countries like India and others. In 2008, more than 180 persons were killed in Bangalore and in 2009, 138 persons were killed in Gujarat, India. Also in 2015, 27 persons died in India after drinking toxic ethanol. In Nigeria, between April and June 2015 a total of 89 persons died following the consumption of locally produced ethanol beverage called kaikai/ogogoro/apeteshi [13]. Osobamiro T, investigated the concentration of methanol in some local and imported alcoholic beverages, Samples A, B, J with concentrations of (21.62 mg/L), (18.25 mg/L), (176.17 mg/L) respectively. All of these concentrations were found to be higher than the permissible limit given by NAFDAC as 0.05mg/l.
Onyenekwe PC, et al. investigated the concentration of acetaldehyde in pito and burukutu and got 12.529 % and 9.348 % respectively, these values were low when compared with other alcoholic distillates. It is important to note that high acetaldehyde concentrations give a pungent irritating odour to the beverage, and can be hazardous to health [14,15]. Chung H, et al. reported that all the alcoholic beverage samples analyzed for this research but for the beer, Korean rice wine and wine containing acetaldehyde, at concentrations between the range from 0.02 to 11.73 mg/L [16]. Osobamiro T, investigated the concentration of ethyl acetate in some local and imported alcoholic beverages, Samples B and G with concentrations of (10.54 mg/l), (30.19 mg/l) respectively, these concentrations were below 150mg/L which also corresponds to the permissible limit set by NAFDAC. Onyenekwe P, et al. investigated the concentration of ethyl acetate present in Pito and Burukutu and it showed 5.692 % and 12.083 % respectively. Chung H, et al. reported the mean values of propanol in whisky and Chinese spirits samples were 701.24 mg/L and 178.57 mg/L respectively. Jude OI, et al. investigated the propanol content in palm wine, molasses and found propanol ranged from 0.05 % to 0.13 % [17]. He also reported the propanol content in spirit distillate to range from 0.06 % to 0.11 %.
Jude OI, et al. also reported the ethyl carbamate content of distillates of cassava recorded an average of 13.44 μg/L, palm wine had an average of 13.75 μg/L and molasses recorded an average of 12.49 μg/L. However, these values were below the permissible limit of the National Agency for Food and Drug Administration and Control (NAFDAC) (1.50 mg/L). Fang F, et al. reported that both EC and precursors (urea and citrulline) were detected in raw spirit after distillation, and found that the EC content of raw spirit of Chinese spirits after the distillation process is 92 μg/L [18]. Milji? UD, et al. reported that in the examination of certain technological procedures during the alcoholic fermentation of plum wine the highest content of benzaldehyde was (3.74 mg/L or 50 mg/L of absolute alcohol) was measured in the experimental wine (K3) [19]. Lu L, et al. reported that benzaldehyde concentration was found to be the highest in (D4) for T1 (535.80 ± 5.60) μg/L and T3 was given as (502.51 ± 116.95) μg/L [20].
Materials and Methods
Reagents
Acetonitrile (C2H3N) was purchased from molychem chemical limited, India J.T. Baker, ethanol, acetaldehyde, methanol, propanol, benzaldehyde, benzene, ethyl acetate was gotten from Accu standard company limited USA. All the reagents and chemicals used were of analytical grade and used without further purification.
Sample extraction and preparation
The alcoholic beverages were refrigerated at 4? until the time for analysis (AOAC official method 5021A). Alcoholic beverage samples were kept at room temperature prior to the analysis time. The sample was extracted by liquid-liquid extraction method using acetronitrile. A portion of the sample was filtered using cellulose microfilter to remove impurities. A 5 mL portion of acetronitrile was placed into a graduated vial and 1.5 mL of the filtered sample was added, it was incubated for 10 mins at 100?. The sample was vortexed for 5 mins then acetronitrile was added to make up to 10 mL. The acetronitrile layer was collected and injected into the GC-FID machine. This was done for each of the samples analyzed in this work. Identification of components, the unknown components of the sample were tentatively identified by comparing the retention times of the chromatogram of the samples to that of the standards. This was done using the peak simple software manufactured by Buck scientific USA.
Preparation of standards
Working standards of the samples were made by serial dilutions (0.5-2.0 mg/L) in 40% acetonitrile. Ethanol standards concentrations ranged from 0.5-2.0 mg/L. It was necessary to use different concentrations for the standards to avoid excessive dilutions which could have resulted in significant error. These serial dilution standards were used to calibrate the Gas Chromatography coupled with Flame Ionization Detector (GC-FID). Ethanol was assumed to be the main ingredient in the samples. Calibration curves were plotted using the peak area ratios of analytes in different standard concentrations to that of the internal standard.
Gas chromatography
Table 1 describes the details of the GC-FID machine used.
| Gas chromatography (A Buck M910) | Features |
|---|---|
| Detector | Flame ionization detector (purchased from Middleburg, Netherlands) |
| Carrier gas | Nitrogen at flow rate 2 mL min-1 |
| Column (polyethylene glycol TPA modified column) | ZB-FFAP, 30 m × 0.32 mm id × 0.25 µm (From Chrompak, Middleburg, Netherlands) |
| Injector | A split/splitless injector |
| Injection and detection temperature | 300? |
| The initial column temperature | Set at 35°C and held for 3 min |
| Ramped temperature | 40°C at 25°C min-1 |
| Held temperature | Held for 2 min, 80°C at 20°C min-1 |
| Held for 4 min, 140°C at 20°C min-1 | |
| Held for 5 min, 220°C at 20°C min-1 | |
| final hold of 1 min | |
| Total run | The total run time was 26 min |
Table 1: Details of the GC-FID machine used.
Quality control
All reagents and chemicals used in this study were analytical grade. The glass wares were washed thoroughly with detergent, rinsed properly with distilled water, and dried in an oven for 3 hours at 50°C before use.
The validation of GC–FID method involves a procedure testing the linearity, precision (repeatability and intermediate precision metrics), LOD, and LOQ as recommended according to previous studies. In order to validate the method and instrument (GC–FID) used for the analysis, a series of standards at different concentrations of the target concentration for parameters was prepared in the range of 0.5–2 mg/L which corresponds to 50%–150% relative to the standards measuring concentration in standard solution. In other to ensure precision, the analysis was carried out in triplicate. After analyzing each preparation in triplicate, a linear regression (calibration graph) analysis was performed on the average peak ratio versus the concentrations of the levels studied.
The correlation coefficient was calculated by plotting component average peak ratio versus component concentrations. Linear regression was applied to the plots and the correlation coefficients for component data were calculated. In order for the test to pass the square of correlation coefficient should not be less than 0.998. The Limit of Detection (LOD) and the Limit of Quantification (LOQ) for each standard solution were defined based on the standard deviation of the calibration curve. The LOD was determined using LOD=3.3 × SD/b, where b is the slope of calibration curve and SD is the standard deviation of the calibration curve while limit of quantification was calculated as LOQ=10 × SD/b.
Recovery analysis
The reason for the recovery analysis was to validate the method of analysis, and it was done by spiking the standard solution of the sample with the contaminant. Spiking is the addition of a known concentration of an analyte to a sample to test if the response to the sample is the same as that from the calibration curve. The difference between the spiked and un-spiked concentration gave the recovery for the amount of spiked sample. The percentage recovery in the spiked sample was calculated using equation 1 (Table 2) [21].
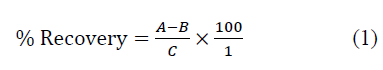
Where;
A=Concentration of spiked
B=Concentration of un-spiked
C=Concentration of the spike added
| Contaminants | Un-spiked concentration mg/L | Mean spiked concentration mg/L | Concentration of spike (mg/L) | % mean recovery | LOD | LOQ |
|---|---|---|---|---|---|---|
| Methanol | 2.5984 | 4.4899 | 2 | 94.6 | 0.009 | 0.03 |
| Propanol | 0.00 | 1.8944 | 2 | 94.7 | 0.012 | 0.04 |
| Ethanol | 24.712 | 26.6289 | 2 | 95.8 | 0.017 | 0.05 |
| Ethyl acetate | 2.2998 | 4.1899 | 2 | 93 | 0.013 | 0.04 |
| Benzaldehyde | 0.0019 | 1.9685 | 2 | 98.3 | 0.007 | 0.02 |
| Benzene | 0 | 1.8949 | 2 | 94.7 | 0.010 | 0.03 |
Table 2: Result of recovery analysis of some organic contaminants in alcoholic beverage sample and LOD and LOQ of each of the contaminants.
Statistical analysis
All alcoholic beverage samples were analyzed, and the levels of ethanol, acetaldehyde, methanol, ethyl acetate, ethyl carbamate, 1-propanol and benzaldehyde were determined and evaluated using both Origin 9.64 and Excel. The results were expressed as the mean, Standard Deviation (SD) and percentage.
Health risk assessment
Health risk assessment was carried out to ascertain possible non-carcinogenic health effects among consumers of these alcoholic beverages produced in Nigeria. HRI greater than one is regarded as a probable potential risk.
Estimated Daily In-take (EDI)
In this study, the Estimated Daily In-take (EDI) for both carcinogenic and non-carcinogenic risks of toxic organic contaminants in alcoholic beverages were estimated using the following equations from Charehsaz M [22].

Where; EDI=Estimated daily intake (mg/kg/day); C=Concentration of contaminant in samples (mg/L) DI=Daily In-take BW=Body Weight (60 kg) The BW was assumed to be 60 kg, as suggested by the United State Environmental Protection Agency
The dose calculation was performed with the standard assumption that the average adult body weight is 60 kg, Daily In-take (DI) was assumed to be=100 ml/day, C is the concentration of the contaminant (mg/L) [23].
Target Hazard Quotient (THQ)
The Target Hazard Quotient (THQ) value is used as a marker, it has been used for expressing non-carcinogenic risk effect. The US EPA mentioned that an index more than one generally indicates a potential for adverse human health effects. However, if the value of THQ is <1, the exposed population are not experiencing any adverse health hazard. If the THQ value=1, the exposed population may experience non-carcinogenic health risks but its reported that as the value increases, the probability increases [24,25]. To calculate and evaluate THQ values an equation was preferred by researchers Ihugba U, et al. and the equation is presented below.

Where; THQ=Target Hazard Quotient EDI=Estimated Daily Intake (mg/kg/day) RfD=Reference oral Dose in (mg/kg/day)
The RfD values are methanol (2 mg/kg/day), ethyl acetate (0.9 mg/kg/day), benzene (0.004 mg/kg/day) and benzaldehyde (0.1 mg/kg/day), they were obtained from data published in the IRIS, whereas that of propanol (2 mg/kg/day) was obtained from the michigan department of environmental quality [26,27].
Results and Discussion
Concentration levels of organic contaminants in all samples
The mean concentration result presented in Table 3 for organic contaminants (methanol, propanol, ethyl acetate, acetaldehyde, ethyl carbamate, benzene, benzaldehyde) in all the samples analyzed shows low concentration of methanol 0.021 mg/L, 0.282 mg/L, 0.491 mg/L, 0.551 mg/L and 0.630 mg/L in samples I, E, A, B and C respectively. The highest concentrations were found in samples G (2.761 mg/L), H (2.627 mg/L), D (2.585 mg/L) and F (1.444 mg/L). The concentration of propanol was found to be 0.507 mg/L and 0.926 mg/L in samples C and D, in the other samples (A, B, E, F, G, H, I) propanol was not detected. Ethyl acetate concentrations ranged from (0.024 mg/L to 3.807 mg/L) with sample (F) having the highest (3.807 mg/L) then samples (I, D, H) with concentrations (3.619 mg/L, 3.305 mg/L, 2.341 mg/L) respectively and the least concentration was recorded in sample (A) at 0.0241 mg/L. Benzene was detected in sample (E) at a concentration of 0.019 mg/L, in other samples benzene was not detected. Benzaldehyde was found in all the samples except for samples (D, G and I), the highest concentration was found in sample (B) at 0.8736 mg/L then 0.2270 mg/L, 0.0210 mg/L for samples (E and F) respectively, its lowest concentration was found in sample A at 0.00028 mg/L. Acetaldehyde and Ethyl carbamate were not detected in the samples analyzed.
| Sample | Methanol | Propanol | Ethyl acetate | Benzaldehyde | Benzene | Acetaldehyde | Ethyl carbamate |
|---|---|---|---|---|---|---|---|
| A | 0.491 ± 0.005 | ND | 0.024 ± 0.000 | 0.000 ± 0.000 | ND | ND | ND |
| B | 0.551 ± 0.0100 | ND | 0.134 ± 0.000 | 0.873 ± 0.005 | ND | ND | ND |
| C | 0.630 ± 0.000 | 0.507 ± 0.000 | 1.3001 ± 0.040 | 0.000 ± 0.000 | ND | ND | ND |
| D | 2.585 ± 0.000 | 0.926 ± 0.000 | 3.305 ± 0.000 | ND | ND | ND | ND |
| E | 0.282 ± 0.003 | ND | 0.029 ± 0.000 | 0.227 ± 0.008 | 0.019 ± 0.000 | ND | ND |
| F | 1.444 ± 0.060 | ND | 3.807 ± 0.019 | 0.021 ± 0.000 | ND | ND | ND |
| G | 2.761 ± 0.030 | ND | 1.355 ± 0.002 | ND | ND | ND | ND |
| H | 2.627 ± 0.003 | ND | 2.341 ± 0.040 | 0.001 ± 0.000 | ND | ND | ND |
| I | 0.021 ± 0.000 | ND | 3.619 ± 0.080 | ND | ND | ND | ND |
| Note: ND: Not Detected | |||||||
Table 3: The concentrations of some organic contaminants in alcoholic beverages expressed in mean and standard deviation of each sample.
Estimated daily in-take
The results obtained for the Estimated Daily In-take (EDI) in mg/kg bw calculated for all the samples and contaminants using the formula stated in equation (3) above ranged from 3.6 × 10-5 to 8.0 × 10-3 mg/kg bw for methanol, 8.5 × 10-4 to 1.5 × 10-3 mg/kg bw in propanol, 3.1 × 10-5 mg/kg bw in benzene, 4.1 × 10-5 to 6.0 × 10-3 mg/kg bw for ethyl acetate and benzaldehyde ranged from 4.7 × 10-7 to 1.5 × 10-3 mg/kg bw. These results are presented in Table 4 below.
| Estimated daily in-take (mg/kg bw) | |||||
|---|---|---|---|---|---|
| Samples | Methanol | Propanol | Ethyl Acetate | Benzene | Benzaldehyde |
| A | 8.0 × 10-3 | - | 4.1 × 10-5 | - | 4.7 × 10s-7 |
| B | 9.2 × 10-4 | - | 2.2 × 10-4 | - | 1.5 × 10-3 |
| C | 1.1 ×10-3 | 8.5 × 10-4 | 2.2 ×10-3 | - | 5.5 ×10-7 |
| D | 4.0 ×10-3 | 1.5 × 10-3 | 5.5 ×10-3 | - | - |
| E | 4.7 × 10-4 | - | 4.9 × 10-5 | 3.1 × 10-5 | 3.7 × 10-4 |
| F | 2.4 × 10-3 | - | 6.0 × 10-3 | - | 3.5 × 10-5 |
| G | 4.6 × 10-3 | - | 2.3 × 10-3 | - | - |
| H | 4.4 × 10-3 | - | 3.9 × 10-3 | - | 3.3 × 10-6 |
| I | 3.6 × 10-5 | - | 6.0 × 10-3 | - | - |
Table 4: Results for the Estimated Daily-Intake (EDI).
Target hazard quotient
Table 5 below shows the results of target hazard quotient.
| Target Hazard Quotient (THQ) | |||||
|---|---|---|---|---|---|
| Samples | Methanol | Propanol | Ethyl acetate | Benzene | Benzaldehyde |
| A | 4.0 × 10-3 | - | 3 × 10-5 | - | 4.7 × 10-6 |
| B | 4.6 × 10-4 | - | 2.5 × 10-4 | - | 1.5 × 10-2 |
| C | 5.0 × 10-4 | 4.2 × 10-4 | 2.4 × 10-3 | - | 5.5 × 10-6 |
| D | 2.0 × 10-3 | 7.7 × 10-4 | 6.1 × 10-3 | - | - |
| E | 2.4 × 10-4 | - | 5.4 × 10-5 | 7.8 × 10-3 | 3.7 × 10-3 |
| F | 1.2 × 10-3 | - | 7.0 × 10-3 | - | 3.5 × 10-4 |
| G | 2.3 × 10-3 | - | 2.5 × 10-3 | - | - |
| H | 2.2 × 10-3 | - | 4.3 × 10-3 | - | 3.3 × 10-5 |
| I | 1.8 × 10-5 | - | 6.7 × 10-3 | - | - |
| RfD (mg/kg/day) | 2 | 2 | 0.9 | 0.004 | 0.1 |
Table 5: Results of the Target Hazard Quotient (THQ).
Discussion
Benzene (C6H6)
Benzene concentration as presented in Table 3 shows that benzene was found in only sample E (0.02 mg/L), which is slightly above the limit set by WHO (0.01 mg/L in drinking water). In other samples benzene was not detected. However, Osobamiro T, reported that benzene was found in only one of the samples analyzed and its concentration was 9.63 mg/L which is almost ten times higher than the permissible limit, this showed higher results when compared to the result gotten in this study Lachenmeier DW, et al. found low concentrations of benzene in alcopops (in 11 out of 12 samples, maximum of 0.44 μg/L) and beer-mixed drinks (in 2 out of 13 samples, maximum of 0.09 μg/L) [28]. These values are lower than the values in this study.
Methanol (CH3OH)
The methanol content was found in all samples analyzed, the highest of which was in sample G (2.7611 mg/L) followed by sample H (2.6272 mg/L) and the least was found in sample I (0.0213 mg/L). This result shows that methanol concentration was above the permissible limit set by NAFDAC (0.05 mg/L) in all the samples except in Sample I. In other works, methanol has also been found, Osobamiro T, reported methanol concentrations analyzed in some alcoholic beverages to range from 176.17 mg/L to 18.25 mg/L which was very much above the limit set by the NAFDAC, these values were very much higher than the values gotten from this study. Methanol which is the lowest molecular weight alcohol, yet the most toxic due to its metabolites formaldehyde and formic acid [5]. In Nigeria, between April and June 2015 a total of 89 persons died following the consumption of locally produced ethanol beverage called kaikai/ogogoro/apeteshi due to methanol poisoning [13]. Also in a similar work, Anarado JO, et al. reported the methanol content in local gins consumed in some selected communities in the Southern Part of Nigeria with concentrations; Nise, Anambra State (0.1005 mg/L), Awba-Ofemilli, Anambra State (11.37 mg/L), Olomoro, Delta Sate (2.4715 mg/L), Okwagbe, Delta State (1.9483 mg/L). Sapele, Delta State (0.200 mg/L contained methanol in concentration above the permissible limit set by the National Agency for Food, Drug Administration and Control (NAFDAC) and European Union at 0.05 mg/L [29].
Ethyl acetate (C4H8O2)
Ethyl acetate was found in all the samples A-I analyzed, the concentration ranged from 0.024 mg/L–3.307 mg/L. These values were within the permissible limit set by NAFDAC at 150 mg/L. Ethyl acetate is one of the major agents responsible for the flavor of alcoholic beverages and their amount determine the quality of the distillate [5]. In similar works, [5] investigated the concentration of ethyl acetate in some local and imported alcoholic beverages, Samples B and G with concentrations of 10.54 mg/L, 30.19 mg/L respectively, these concentrations were below 150 mg/L which also corresponds to the permissible limit set by NAFDAC.
Propanol (C3H8O)
Propanol was found in only 2 samples (C and D) at concentrations of 0.5079 mg/L and 0.9264 mg/L respectively, these values are less than the permissible limit set by EU for higher alcohols at 0.005 g/L. In related works, Onyenekwe PC, reported the percentage concentration of propanol in locally manufactured pito and burukutu and the result showed that burukutu had a higher percentage of propanol (11.586 %) while pito had 7.675 % of propanol present. Also, Chung H, et al. reported the mean values of propanol in whisky and Chinese spirits samples were 701.24 mg/L and 178.57 mg/L respectively, these values are higher when compared to the results obtained in this work.
Benzaldehyde (C6H5CHO)
The benzaldehyde concentrations as presented in Table 3 showed that benzaldehyde was found in 6 out of 9 samples analyzed, the concentrations ranged from 0.0033–0.8736 mg/L. These values are lower than the permissible limit set by EU at 0.005 g/L for aldehydes. From literature, benzaldehyde has been found in other samples analyzed. Milji? UD, et al. reported that the highest content of benzaldehyde in plum wine was (3.74 mg/L or 50 mg/L of absolute alcohol) was measured in the experimental wine (K3). Also,
Acetaldehyde and ethyl carbamate which are known carcinogens as declared by IARC was below the detection limit in the samples analyzed in this work.
Health risk assessment
The Estimated Daily Intake (EDI) and target hazard quotient (THQ) of some alcoholic beverage samples.
The Estimated Daily Intake (EDI) as presented in Table 4. EDI is the product of concentration of the organic contaminants, the daily intake divided by average body weight. The Estimated daily intake for all samples and analytes are given in Table 4. The values for methanol ranged from 3.6 × 10-5–8.0 × 10-3 mg/kg bw, propanol ranged from 8.5 × 10-4–1.5 × 10-3 mg/kg bw, ethyl acetate ranged from 4.9 × 10-5–6.0 × 10-3 mg/kg bw, benzaldehyde ranged from 5.5 ×10-7–1.5 × 10-3 mg/kg bw for the non-carcinogenic human health risk assessment, while the carcinogenic human healthrisk assessment showed the Estimated Daily Intake (EDI) to be 3.1 × 10-5 mg/kg bw in benzene. In a related work,Manolis K, et al. reported the risk assessment of some bottled and in-bulk spirits to have an EDI for bottled spirit of1.00 mg/kg bw for methanol, 0.60 mg/kg bw for ethyl acetate and 0.24 mg/kg bw for propanol while for the bulk spirit,methanol was 1.12 mg/kg bw, ethyl acetate 1.53 mg/kg bw and propanol 0.25 mg/kg bw.
Also, Nili-Ahmadabadi A, et al. reported the Estimated Daily Intake (EDI) in methanol found in some herbal distillates to range from 0.025 mg/kg bw to 0.033mg/kg bw if the daily in-take was assumed to be 10 ml [30].
The result of the Target Hazard Quotient (THQ) is presented in Table 5. The THQ which is a non-carcinogenic biomarker was estimated by dividing the EDI with the Reference Doses (RfD). The THQ values for methanol ranged from 1.8 × 10-5–4.0 × 10-3, propanol had values ranging from 2 × 10-4–7.7 × 10-4, ethyl acetate had values ranging from 5.4 × 10-5–7.0 × 10-3 and benzaldehyde had values ranging from 5.5 × 10-6–1.5 × 10-2 while the carcinogenic compound present has a THQ of 7.8 × 10-3. The values for all the contaminants considered in this work was found to be less than 1 which is considered safe for human health [26]. In related works, Manolis K, et al. reported the risk assessment of some bottled and in-bulk spirits to have HRI for non-carcinogenic compounds for bottled spirits to be 0.50 for methanol, 0.67 for ethyl acetate and 0.12 for propanol while for the in bulk spirits, methanol was 0.56, ethyl acetate 1.69 and propanol 0.12. This shows that only ethyl acetate found in bulk spirit was above 1 indicating possible side effects according to the US National Library of Medicine. These values were however higher than the results gotten from this work. Nili-Ahmadabadi A, et al. reported the HRI values in methanol to range from 0.012 to 0.026, these values are all less than 1 which means they are safe if these beverages are consumed at 10 mL (Daily intake), there is however a possibility that if these herbal drinks are consumed in higher quantity the HRI may be above 1 and may have health implications. In a related work, Jiang Q, et al. reported in his work that the methanol Health Index (HI) of various alcoholic beverage consumers were all below one. The average methanol HI of consumers were ranged between 0.005 to 0.079 [31,32].
Conclusion
The result shows that methanol was found in all samples and were above the permissible standard set by NAFDAC (0.05 mg/L) in all the samples except in sample I. Propanol was found in 2 of the samples and the concentrations were less than the permissible limit given by EU as 0.005 g/L for higher alcohols. Ethanol was found to be the with the highest concentrations in all the samples, the IARC has already declared ethanol as a group 1 carcinogen. Ethyl acetate was found in all the samples analyzed, the concentrations were below the permissible limit set by NAFDAC at 150 mg/L. Benzene was found in only sample E, which was slightly above the limit set by WHO as (0.01 mg/L). Benzaldehyde was found in 6 samples; the concentrations were lower than the permissible limits set by EU as (0.005 g/L) for aldehydes. Acetaldehyde and ethyl carbamate were below the detection limit in the samples analyzed in this work.
The human health risk assessment carried out in this work showed the values of the estimated daily-intake to range from 5.5 × 10-7–8.0 × 10-3 mg/kg bw. Target hazard quotient ranged from 5.5 × 10-6–7.8 × 10-3. All samples had THQ values for the organic contaminants to be ?1 which means it is safe for human health.
This work has shown that most of our locally manufactured alcoholic beverages (Spirit) contain organic contaminants, either below or above the permissible limit set by the regulatory agencies. The effects of drinking alcoholic beverages with contaminants present may not show forth immediately as they have the ability to bio-accumulate in the body and this means the effects will most likely be in the future.
It could be concluded that some poor people in Nigeria could be at risk of these contaminants poisoning as these locally produced alcoholic beverages are the most popular drinks among the poor populace in Nigeria. This work is of great relevance to the producers of these beverages, the consumers of the products, the regulatory/law enforcement agencies and the society at large. It will help to monitor the production of these alcoholic beverages, the contaminants present and their concentrations in which they are present. By so doing, this will help to boost the quality of the manufacturing of our locally produced alcoholic beverages, it will also help to minimize the effect and health risk of the contaminants by educating the public mostly the consumers of the effects/risks of these contaminants on the human health. Studies have shown that long-term use of alcohols in excessive quantities is capable of damaging nearly every organ and system in the body.
References
- Vara S., et al. Natural preservatives for nonalcoholic beverages. In Preservatives and preservation approaches in beverages . Academic Press. 2019:179-201
[Crossref]
- Pang XN, et al. J Food Prot. 2017;80(3):431-442.
[Crossref] [Google Scholar] [PubMed]
- La Villa J., The wine, beer and spirits handbook: A guide to styles and service. John Wiley and sons. 2010.
- Chung H, et al. J Korean Soc App Biol Chem. 2015;58:423-432.
- Osobamiro T. American-Eurasian J Sci Res. 2013;8(1):53-56.
- Izah SC, et al. Toxics. 2016;5(1):1.
[Crossref] [Google Scholar] [PubMed]
- Ubuoh EA, et al. Int J Mat Sci. 2013;1(5):90-95.
- Salako SG, et al. British J App Sci Technol. 2016;12(6):1-8.
- World Health Organization. Global status report on alcohol and health. 2014.
- Rather IA, et al. Frontiers Pharmacol. 2017;8:308465.
[Crossref] [Google Scholar] [PubMed]
- Wi?niewska P, et al. Crit Rev Anal Chem. 2015;45(3):201-225.
[Crossref] [Google Scholar] [PubMed]
- Katarzyna P, et al. Alcoholic Bev. 2019;7:65-111.
- Ohimain EI. Springerplus. 2016;5(1):1607.
[Crossref] [Google Scholar] [PubMed]
- Onyenekwe PC, et al. Nat Prod Res. 2016;30(5):558-564.
[Crossref] [Google Scholar] [PubMed]
- Geroyiannaki M, et al. Food Cont. 2007;18(8):988-995.
- Chung H, et al. J App Biol Chem. 2012;55(3):141-148.
- Iwouno JO, et al. Asian Food Sci J. 2019;7(3):1-2.
- Fang F, et al. Food Biosci. 2018;23:137-141.
- Milji? UD, et al. J Processing Energy Agri. 2019;23(1):46-49.
- Lu L, et al. Food Qual Safety. 2022;6:fyac037.
- Ucheana IA, et al. Int J Environ Analytical Chem. 2022:1-20.
- Charehsaz M, et al. Hum Exp Toxicol. 2021;40(8):1241-1249.
[Crossref] [Google Scholar] [PubMed]
- Kokkinakis M, et al. Toxicol Rep. 2020;7:1057-1065.
[Crossref] [Google Scholar] [PubMed]
- Muñoz O, et al. Food Chem Toxicol. 2017;109:1125-1134.
[Crossref] [Google Scholar] [PubMed]
- Ihugba UA, et al. Am J Environ Protect. 2018;6(1):22-27.
- United State Environmental Protection Agency. IRIS Toxicological Review of Methanol (External Review Draft; December 2009). U.S. Environmental Protection Agency, Washington, DC, 2010.
- Michigan Department of Environmental Quality (MDEQ). Chemical Update Worksheet. RRD Toxicology Unit. Propyl Alcohol. 2015.
- Lachenmeier DW, et al. Food Chem Toxicol. 2008;46(8):2903-2911.
[Crossref] [Google Scholar] [PubMed]
- Anarado JO, et al. J Chem Soc Nigeria. 2019;44(4):671-675.
- Nili-Ahmadabadi A, et al. J App Pharmaceut Sci. 2016;6(7):49-52.
- Jiang Q, et al. J Food Nutr Disor. 2016;5:4.
- Caan W., et al. Drink, drugs and dependence: From science to clinical practice. Routledge. 2003.
[Crossref]
