Original Articles: 2022 Vol: 14 Issue: 8
Kinetics and mechanistic study of oxidation of biotin (vitamin b7) by vanadium (V) an insulin mimic compound at low pH
Received: 27-Jan-2021, Manuscript No. JOCPR-19-3717; Editor assigned: 01-Feb-2021, PreQC No. JOCPR-19-3717 (PQ); Reviewed: 15-Feb-2021, QC No. JOCPR-19-3717; Revised: 02-Aug-2022, QI No. JOCPR-19-3717; Manuscript No. JOCPR-19-3717 (R); Published:30-Aug-2022, DOI: 10.37532/09757384.2022.14(7).001.
Abstract
Oxidation of biotin (vitamin H) by vanadium (V) was studied under pseudo-first order conditions in the temperature range 293K-318K at low pH (1.6-2.4), where vanadium (V) taken excess over biotin. The reaction was proceed through an intermediate to form the complex. With increase in pH the pseudo-first order rate constant decreases, with decrease of dielectric constant of the medium. The ionic strength had no significant effect on the rate of the reaction. There is a probable mechanism which leads to the oxidation of biotin to biotin sulfone. Electron transfer from biotin to V(V) center lead to the release of reaction product (biotin sulfone), VO2+ and a molecule of water. The activation parameters were determined and are consistent with the proposed mechanism for the formation of biotin sulfone and V(IV). Formation of the product was characterized by UV-Visible spectra, 1H-NMR spectra, Mass spectra and Elemental analysis which favors for biotin sulfone.
Keywords
Biotin; Vanadium. Kinetic study; Oxidation; Pseudo-first order reaction
Introduction
Biotin, Hexahydro-2-oxo-1H-thieno (3,4-d) imidazole-4-pentanoic acid (Figure 1) is a water-soluble B-complex vitamin. It is a heterocyclic, sulphur containing monocarboxylic acid also known as vitamin B7 or vitamin H or coenzyme R. Biotin is a important cofactor in the catalysis of essential metabolic reactions to synthesize fatty acids, in gluconeogenesis. Biotin free and bound biotin are readily oxidized by oxidizing agents to form oxy derivatives.3 Authors reported a kinetic spectrophotometric method of biotin for determination of the biological products in pharmaceutical dosage forms and human serum. Biotin is an important component of enzymes involved in metabolizing fats and carbohydrates, influencing cell growth, and affecting amino acids involved in protein synthesis. Biotin assists in various metabolic reactions involving the transfer of carbon dioxide. It may also be helpful in maintaing a steady blood sugar level. It is important in fatty acid synthesis, branched chain amino acid catabolism and gluconeogenesis [1].
Vanadium is an essential trace element found in all living organisms. Vanadium ion acts as an enzyme cofactor found in possible tunicates and also in mammals. The reduction of V(V) by oxalic acid, hydroquinone, hypophosphorous acid proceeds through a complexation pre-equilibrium followed by an inner sphere one electron transfer path ways [2,3]. Vanadium (IV) L-ascorbate complexes were formed by the reduction of V(V) by L-ascorbic acid at low pH under physiological conditions used as insulin-enhancing agents for treatment of diabetes. The reduction of V(V) with NADPH in the presence of EDTA takes place readily in acidic media to produce V(IV). 29-Vanadium is found in two oxidation forms, V(V) and V(IV). V(V) is the most abundance and toxic species. Vanadium is essentially required element that helps in carbohydrate metabolism preventation of some heart diseases [4].Vanadium complex can act as oxidizing agent, insulin-mimic drug, dehydrating catalyst. Vanadium form complexes in -2 to +5 oxidation states. But +3, +4, +5 oxidation state of vanadium found in living organism. The redox chemistry of vanadium (V) was reported by inorganic and biological reducing agents. At low pH, vanadium exists as cis-dioxovanadium, VO2+, however as the pH is increased, the most prevalent forms are vanadate, H2VO4-or decavanadate H3V10O28.
Materials and Methods
All the chemicals used were AnalR grade and were used without further purification. Biotin was received from SRL company [5]. Solutions of different concentrations were prepared by proper dilution of stock solution. Doubly distilled water was used for the preparation of solutions throughout the experiment. Absorbance measurement have been done by using Agilent Cary 100 UV-Visible spectrophotometer [6]. The pH of the solution was measured by using Electronics India digital pH meter model-101.
Kinetic study
For kinetic measurements fresh solutions were prepared. The kinetics of the reaction between biotin and ammonium metavanadate was studied under pseudo-first order conditions in the temperature range 293K-318K at low pH (1.6-2.4), where vanadium (V) taken excess over biotin. The change in absorbance was measured by following the decrease in absorbane at λmax=260 nm. The pH of the solution was adjusted by using HCl or NaOH and was measured before and after the kinetic study. No significant change was observed [7].
Stoichiometry and product analysis
For determining stoichiometry, different reaction mixtures containing various concentration of biotin and ammonium vanadate in a molar ratio of 1:10 were warmed at 318K at I=0.5 mol dm-3 and pH=2.0 for 4 h till the reaction was completed. The unreacted vanadium concentration was estimated spectrophotometrically. Biotin reduces aqueous vanadium (V) according to the following equation:
Biotin + VO2+ + 2H+=Biotin sulfone + VO2++H2O
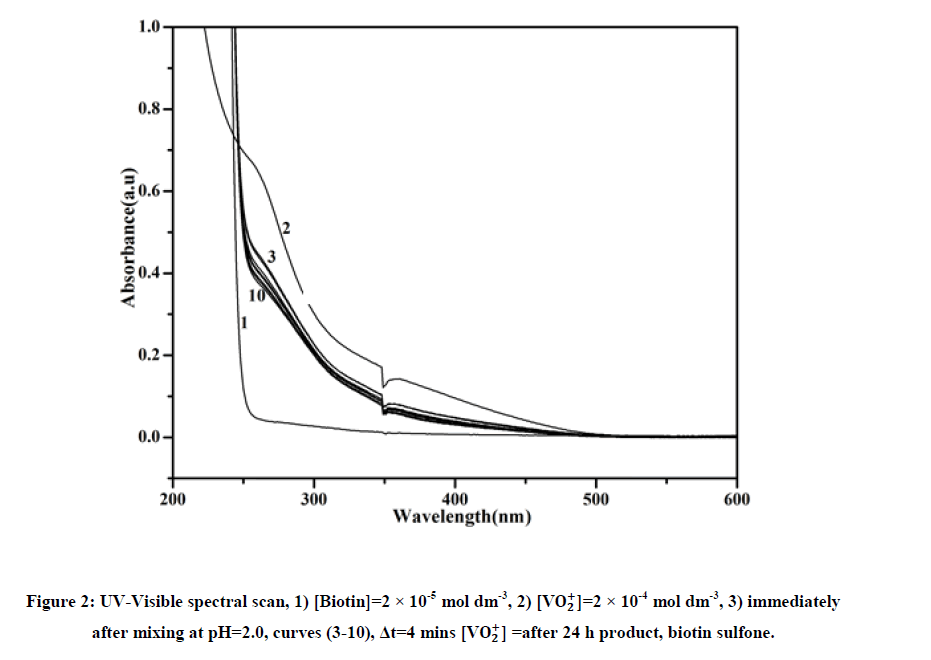
From the above experiment, it was observed that 1 mole of biotin reacted with one mole of vanadium to generate product. The stoichiometry was found to be 1:1 as depicted in equation. In order to get the reaction product, ammonium metavanadate (0.2 mol) and biotin (0.2 mol) was dissolved in 20 ml of water at pH=2.0 and were warmed and allowed to complete the reaction [8,9]. From the solution metal ion was removed through cation exchange resin. The solution was slowly evaporated to concentrate the solution, then it was kept overnight, a brown crystalline product was formed. It was washed with ethanol and dried in desiccators containing silica gel. The yield of the product was 75%. UV-Visible spectra of product shows λmax=275 nm (Figure 2). The product was confirmed by FTIR spectra, 1H-NMR spectra, Mass spectra and elemental analysis as biotin sulfone [10].
Results and Discussion
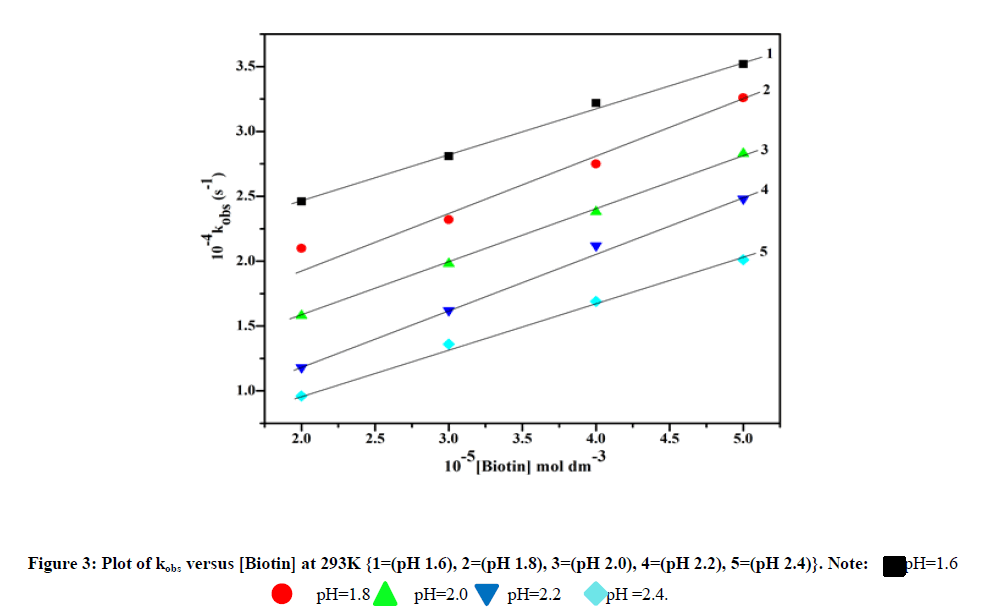
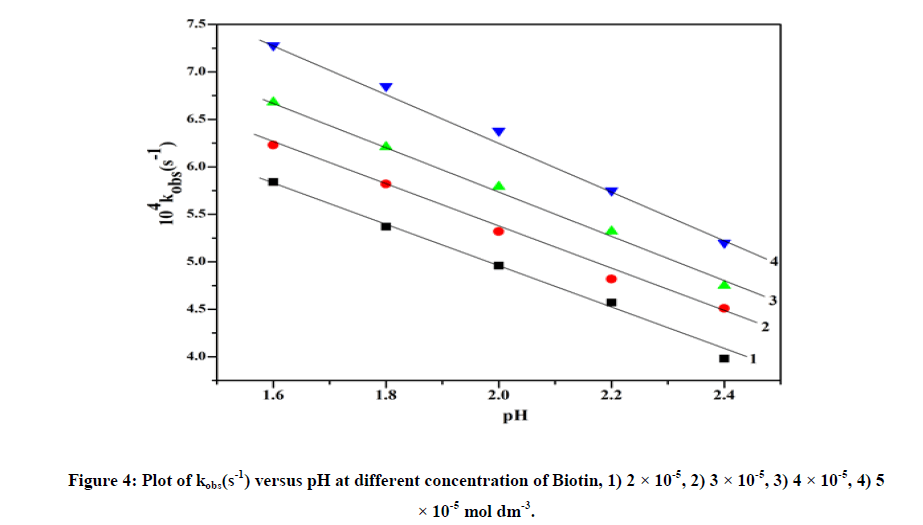
The redox reaction between biotin and vanadium (V) was studied over the range 2.0 ≤ 105 [Biotin] ≤ 5.0, 1.7 ≤ pH ≤ 2.5, 293K ≤ T ≤ 313K and ionic strength (I)=0.5 mol dm-3 (Table 1). The aqueous solution of ammonium vanadate was yellow in color due to formation of active species VO2+ which shows a peak at λmax=264 nm [11]. Biotin shows peak in the UV-Visible spectra at 204 nm. When two solutions are mixed at pH=2.0, yellow color slowly changes to green color indicated by spectral change (λmax=260 nm). Time scan spectra shows decrease of absorbance at λmax=260 nm with time (Figure 2). After a long interval of time (24 h) these peaks disappeared and new peak appeared at λmax=335 nm corresponding to V(IV) aquo (Figure 2). The plot of kobs versus [Biotin] was linear (Figure 3) with a significant intercept indicating first order dependence on biotin.
| 105 [Biotin] mol dm-3 | pH | 104 kobs (s-1) | |||
|---|---|---|---|---|---|
| 293K | 298K | 313K | 318K | ||
| 1 | 1.6 | 2.46 | 3.24 | 4.43 | 5.84 |
| 1.8 | 2.1 | 2.56 | 4.11 | 5.37 | |
| 2 | 1.58 | 1.96 | 3.72 | 4.96 | |
| 2.2 | 1.18 | 1.45 | 3.24 | 4.57 | |
| 2.4 | 0.96 | 1.06 | 2.58 | 3.98 | |
| 2 | 1.6 | 2.81 | 3.72 | 4.89 | 6.23 |
| 1.8 | 2.32 | 3.1 | 4.55 | 5.82 | |
| 2 | 1.98 | 2.46 | 4.06 | 5.32 | |
| 2.2 | 1.62 | 1.87 | 3.68 | 4.82 | |
| 2.4 | 1.36 | 1.48 | 3.04 | 4.51 | |
| 3 | 1.6 | 3.22 | 4.14 | 5.37 | 6.68 |
| 1.8 | 2.75 | 3.5 | 4.98 | 6.21 | |
| 2 | 2.38 | 2.98 | 4.55 | 5.79 | |
| 2.2 | 2.12 | 2.35 | 4.17 | 5.32 | |
| 2.4 | 1.69 | 1.85 | 3.55 | 4.75 | |
| 4 | 1.6 | 3.52 | 4.48 | 5.78 | 7.28 |
| 1.8 | 3.26 | 3.99 | 3 | 6.85 | |
| 2 | 2.83 | 3.41 | 4.96 | 6.38 | |
| 2.2 | 2.48 | 2.93 | 4.55 | 5.75 | |
| 2.4 | 2.01 | 2.29 | 4.08 | 5.2 | |
Table 1: Pseudo-first order rate constant for redox reaction of vanadium (V) by biotin at pH (1.6-2.4), temperatures (293K-318K) and biotin concentrations (2.0 × 10-5-5.0 × 10-5) mol dm-3.
Effect of pH
When pH of the reaction solution varies from 1.6 to 2.4, it has been shown that pseudo-first order rate constant (kobs) decreases (Figure 4). Above pH>3.0, the kinetic study was prevented, so rate can not be measured [12].
Ionic strength effect
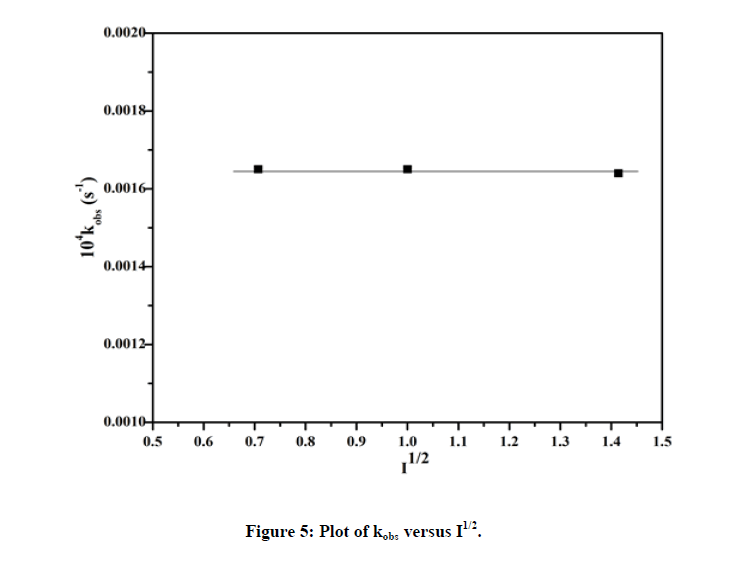
The effect of ionic strength (I) was calculated on the variation of NaNO3 from 0.1 mol dm-3 to 0.5 mol dm-3 at pH=2.4 (Table 2), keeping all other conditions constant. The plot of kobs versus (√I) (Figure 5) indicates the negligible effect of ionic strength.
| Ionic strength I (mol dm-3) |
vI | 104 kobs (s-1) |
|---|---|---|
| 0.5 | 0.707 | 1.38 |
| 1.0 | 1.0 | 1.37 |
| 2.0 | 1.414 | 1.37 |
Table 2: Pseudo-first order rate constants with variation of ionic strength.
Solvent polarity effect
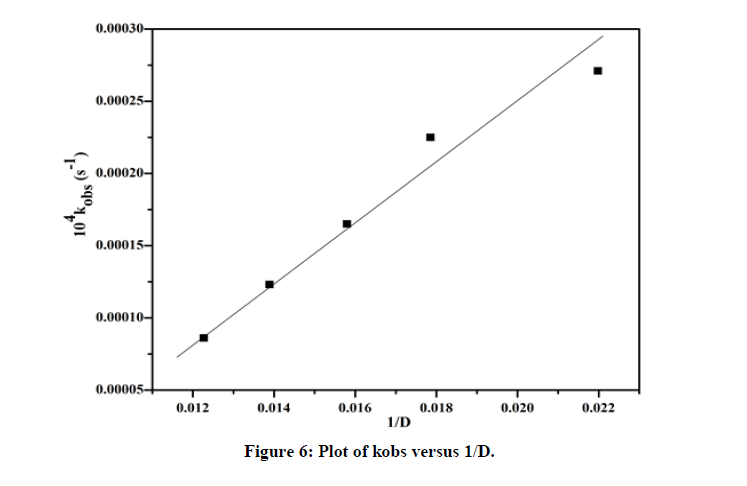
At constant temperature (i.e. 303K), the dielectric constant of the solvent was considered by varying acetic acid from 20% to 60% keeping all other conditions constant [13,14]. 104 kobs (s-1) changed from 0.65 to 2.71 when the dielectric constant changes from 20% to 60% (Table 3). Plot of log kobs versus D-1 (Figure 6) is a straight line with a positive slope (R2=0.99) indicating ion dipolar interaction in the rate determining step following Amies concept.
| % of CH3COOH | Dielectric constant (D) | 104 kobs (s-1) |
|---|---|---|
| 20 | 81.5 | 0.65 |
| 30 | 72 | 1.47 |
| 40 | 63.3 | 1.86 |
| 50 | 56 | 2.25 |
| 60 | 45.5 | 2.71 |
Table 3: Pseudo-first order rate constants with different dielectric constants (D) for the oxidation of biotin.
The interventation of free radical was examined by adding acrylonitrile to the reaction mixture and keeping it for 2 h at room temperature [15]. Upon dilution with methanol, the reaction mixture did not show any precipitate indicating no intervention of free radicals during the reaction [16].
Temperature effect
The rate of the reaction was measured at four different temperatures 293K, 298K, 308K and 313K under varying biotin concentrations and varying pH=1.6 to 2.4. The rate constant was found to increase with increasing temperature (Table 1).
Mechanism of the reaction
Basing on the above experimental facts, stoichiometry, identification of product, the probable mechanism may be delineated as in Scheme I [17].
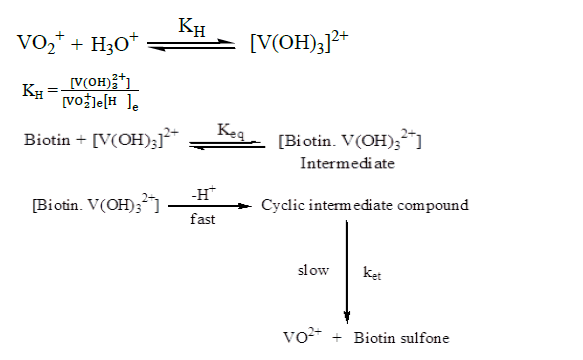
The rapid formation of the intermediate was followed by a slower step intramolecular electron transfer to produce vanadium (IV) and biotin sulfone
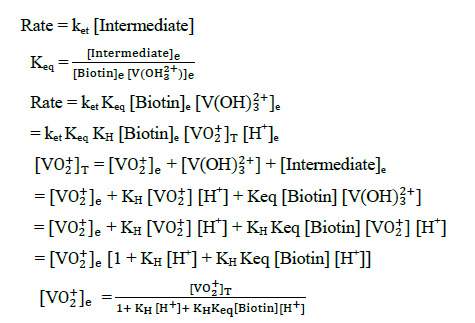
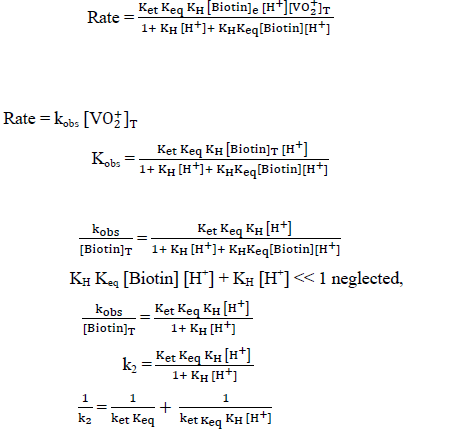
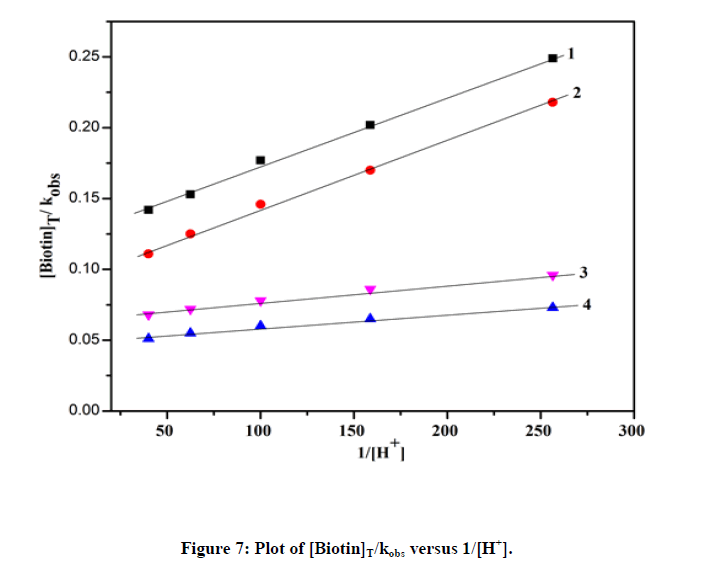
The plot of 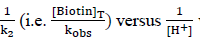 was found to be linear (Figure 7) with slope value 1/ ket Keq KH. Inversion of intercept produces composite rate constant i.e. (ket Keq=k' ) and the ratio of intercept to slope gives the value of KH (Table 4).
was found to be linear (Figure 7) with slope value 1/ ket Keq KH. Inversion of intercept produces composite rate constant i.e. (ket Keq=k' ) and the ratio of intercept to slope gives the value of KH (Table 4).
| Temperature (K) | K | KH |
|---|---|---|
| 293 | 10.948 | 190.807 |
| 298 | 15.849 | 127.75 |
| 313 | 21.234 | 340 |
| 318 | 21.617 | 472.5 |
Table 4: Calculation of compositerate constant for electrontransfer reaction equilibrium constant (KH) and activation parameter sand thermodynamic parameters.
The final oxidation product of biotin gives biotin sulfone and vanadium (IV). As compared to several investigation, the results are in good agreement. The enthalpy of activation ΔH≠ and entropy of activation ΔS≠ are obtained by following the Eyring equation.
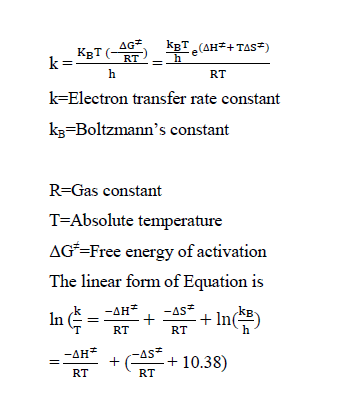
A graph is plotted between log (k/T) versus 1/T. From the slope, the value of enthalpy of activation and from the intercept the value of entropy of activation were calculated[18]. It has been found that, ΔH≠ = 32.68 kJ mol-1 and ΔS≠ = -112.72 JK-1 mol-1. Using the value of ΔH≠ and ΔS≠, the free energy of activation is calculated as ΔG≠(298) 33.62 kJ mol-1
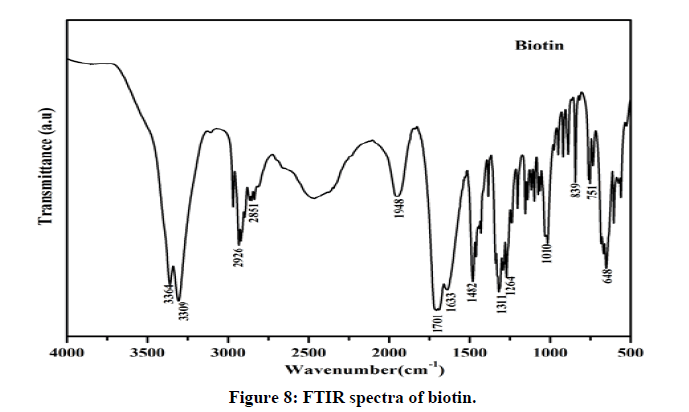
The FTIR spectra of biotin (Figure 8) showed characteristic bands at 3364 cm-1 for OH stretching of COOH group, for imidazole ring (3306 cm-1), methylene (2926 cm-1 and 2851 cm-1), carbonylgroup (1701 cm-1 and 1633 cm-1) and skeletal vibrations of C-C moieties (1311 cm-1 and 1264 cm-1), C-S stretching vibration (648 cm-1).
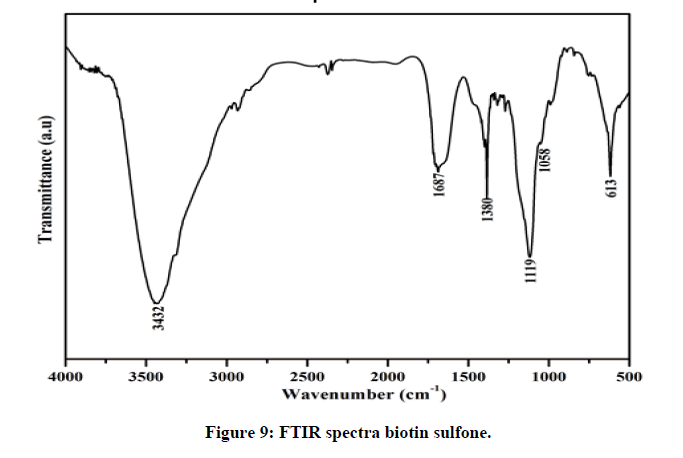
FTIR spectra of the oxidation product of biotin (Figure 9) shows a broad peak at 3432 cm-1 is due to O-H stretching. In ammonium vanadate-biotin complex, the absorption band at 1120 cm-1 corresponds for the formation of (O=S=O) bonding does no longer appear in free biotin spectra, which proves that the synthesized product was named as biotin sulfone [19]. A lower value appeared at 613 cm-1 compared to free biotin for C-S stretching [20,21]. Similar product have been reported by others using hydrogen peroxide as oxidants [22]. It is further supported by characteristic peak at λmax=254 nm in UV-Visible spectra.
1H-NMR spectra
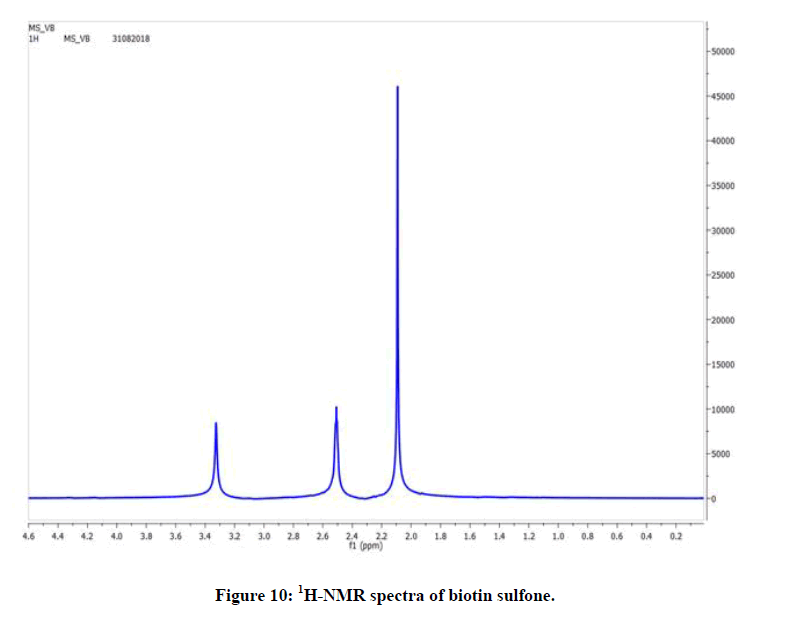
1H-NMR of the oxidation product of biotin (Figure 10) shows peaks in the range δ (1.9-2.2) for methylene groups (2H, CH2), δ (2.4- 2.6) for methane protons and δ (3.2-3.4) for ureic protons [23,24].
Mass spectra
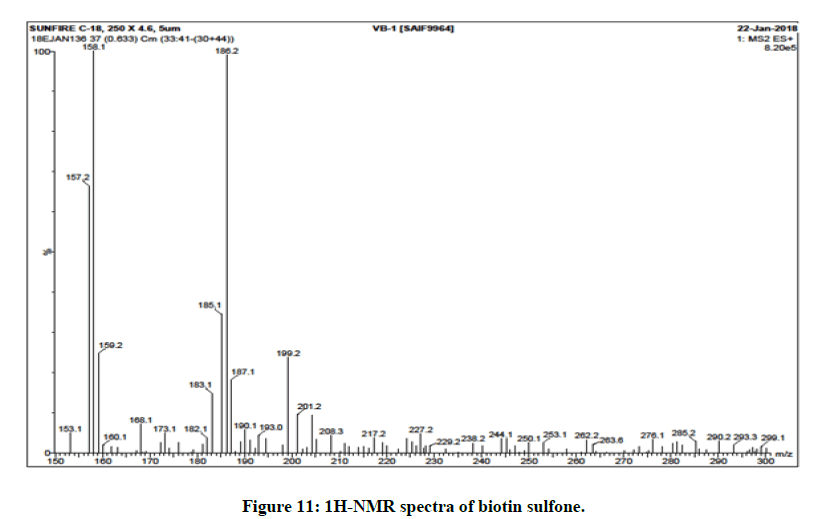
A simple, specific and sensitive analytical method for the mass determination of the product biotin sulfone had been analysed and it was also satisfied (Figure 11) [25,26]. Biotin sulfone shows a base peak corresponding to the protonated molecular ion [M+H]+ (m/z=186).
This is for 4-pentanoic acid with sulfone group [27]. The m/z value at 276 corresponds to the formation of biotin sulfone [28]. After the loss of hexahydro-2-oxo-1H-thieno (3,4-d) imidazole from biotin sulfone the m/z value arises at 157.
Elemental analysis
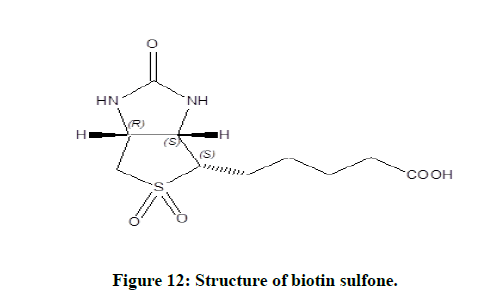
Elemental analysis (CHNO) of the isolated product was done using CHN analyser. The estimation of vanadium metal was done through AAS. The C, H, N and O elemental analysis shows that the percentage of carbon (43.10), hydrogen (5.36), nitrogen (9.85) and oxygen (28.74) were corresponding to the theoretical percentage values of the complex (Table 5). The above elemental analysis supports for the formation of biotin sulfone (Figure 12).
| Element | % present |
|---|---|
| Carbon | 43.10 (43.48)* |
| Hydrogen | 5.36 (5.80)* |
| Nitrogen | 9.85 (10.14)* |
| Sulphur | 28.74 (28.98)* |
| *Represents theoretical values | |
Table 5: Percentage of the elements in vanadium-biotin complex.
Conclusion
Oxidation of biotin to biotin sulfone has been performed efficiently by vanadium (V) compound. The reactions were studied spectrophotometrically between pH 1.6-2.4 in the temperature range 293-313K. The reaction is first order in both biotin and vanadium (V) concentrations. As the pH increases from 1.6 to 2.4, the pseudo-first rate constant decreases with decrease of the dielectric constant and the rate of the reaction increases. Ionic strength has no effect on such reaction. The formation of sulfone established the thioether nature of the sulfur atom and it is therefore concluded that biotin is a monocarboxylic acid containing a cyclic urea structure with the sulfur atom in thioether linkage.
Acknowledgement
We authors thank to Central Drug Research Institute, Lucknow for supporting NMR study and CHN analysis and Central Drug Research Institute, Chandigarh for Mass spectra bacterial strains tested (E. coli and S. aureus). Gram-negative bacterium E. coli was shown more sensitive than Gram positive bacterium S. aureus. Copper complexes 2 and 3 showed good inhibition on E. coli and S. aureus strains (MIC of 3 mg/ml). The combination of lactic acid copper complexes enhanced antibacterial action leading to synergistic effect. Our findings suggested the possibilities of use of copper complexes or their combinations at industrial scale as additives for the preservation of food, and pharmaceutical, cosmetic or cleaning purposes.
References
- Che P, Weaver LM, Wurtele ES. Plant Physiol. 2003;131(3):1479-1486.
- Holmberg A, Blomstergren A, Nord O, et al. Ectrophoresis. 2005;26(3):501-510.
- Ramalingaiah RV, Jagadeesh, Puttaswamy. J Mol Catal A Chem. 2007;265(1-2):70-79.
- Upadhya K, Khattak IK, Mullah B. Nucleos Nucleot Nucl. 2005;24(5-7):919-22.
- Walash MI, Rizk M, Sheribah ZA, et al. Int J Biomed Sci. 2008;4(3):238.
- Espinosa-Mansilla A, Valenzuela MA, de la Peña AM. et al. Analytica Chimica Acta. 2001;427(1):129-36.
- Ross AC, Caballero B, Cousins RJ. Modern Nutr Health Dis. 2020.
- Pacheco-Alvarez D, Solórzano-Vargas RS, del R??o AL. Arc Med Res. 2002;33(5):439-447.
- Baran EJ. J Braz Chem Soc. 2003;14:878-888.
- Kütter VT, Montes-Bayón M, Sella SM, et al. J Braz Chem Soc. 2014;25:1116-1123.
- Rehder D. Metallomics. 2015;7(5):730-742.
- Crans DC. Pure Appl Chem. 2005;77(9):1497-527.
- Rehder D. J Inorg Biochem. 2000;80(1-2):133-6.
- Kundu S, Maity S, Maity AN, et al. Dalton Trans. 2013;42(13):4586-601.
- Wever R, Kustin K. Adv Inorg Chem. 1990;35:81-115.
- Littlechild J. Curr Opin Chem Biol. 1999;3(1):28-34.
- Baran EJ. J Braz Chem Soc. 2003;14:878-888.
- Jabeen M, Ali S, Shahzadi S, Shahid M, et al. J Gen Chem. 2017;87(3):530-538.
- Bruyère VI, Rodenas LA, Morando PJ, et al. J Chem Soc Dalton trans. 2001(24):3593-3597.
- Wells CF, Kuritsyn LV. J Chem Soc (A). 1970:1372-1376.
- Cooper JN, Hoyt HL, Buffington CW, et al. J Phys Chem. 1971;75(7):891-894.
- Wilkins PC, Johnson MD, Holder AA, et al. Inorg Chem. 2006 Feb 20;45(4):1471-1479.
- Kanamori K, Sakurai M, Kinoshita T, et al. J Inorg Biochem. 1999;77(3-4):157-161.
- Islam MK, Tsuboya C, Kusaka H, et al. Biochimica Et Biophysica Acta. 2007;1770(8):1212-1218.
- Despras G, Robert R, Sendid B, et al. Bioorg Med Chem. 2012;20(5):1817-1831.
- Collot M, Sendid B, Fievez A, et al. J Med Chem. 2008;51(19):6201-6210.
- Balan V, Petrache IA, Popa MI, et al. J Nanoparticle Res. 2012;14(2):1-4.
- Hofmann K, Melville DB, Du Vigneaud V. J Biol Chem. 1941;141:207-214.