Original Articles: 2025 Vol: 17 Issue: 1
Kinetic Modelling and Sensitivity Analysis of Acetylation Unit of Continuous Acetaminophen Production Process: Aspen® Plus Simulation Study
Muhammad Alhassan Adam, Usman Aliyu El-Nafaty, Saidat Olanipekun Giwa, Ahmed Mohammed Inuwa*
Department of Chemical Engineering, Abubakar Tafawa Balewa University, Bauchi, Nigeria
*Corresponding Author:
- Ahmed Mohammed Inuwr
Department of Chemical Engineering, Abubakar Tafawa Balewa University, Bauchi, Nigeria
Received: 22-Dec-2023, Manuscript No. JOCPR-23-110782; Editor assigned: 27-Dec-2023, PreQC No. JOCPR-23-110782 (PQ); Reviewed: 10-Jan-2024, QC No. JOCPR-23-110782; Revised: 22-Jan-2025, Manuscript No. JOCPR-23-110782 (R); Published: 29-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).221.
Citation: Inuwa AM, et al. 2025. Kinetic Modelling and Sensitivity Analysis of Acetylation Unit of Continuous Acetaminophen Production Process: Aspen® Plus Simulation Study. J Chem Pharm Res., 17:221.
Copyright: ©2025 Inuwa AM, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Pharmaceutical companies, who now rely on batch technology, are starting to move towards Continuous Pharmaceutical Manufacture (CPM) as a result of recent advancements in pharmaceutical manufacturing techniques that have demonstrated the promise of continuous production. Methods of production that are continuous have advantages in terms of effectiveness, price, dependability, and quality. This study conducted using Aspen Plus version 11.0 to developed simulation study on the acetylation reaction unit, which is one of the most important units the continuous acetaminophen production process. The developed acetylation reactor kinetic simulation model using Aspen Plus under the continues acetaminophen synthesis was validated using UNIQUAC-ideal as fluid package, the result of the validation was successful with 0.36% error, which is acceptable as it was less than unity. Also, the results of the sensitivity analysis revealed that 1.0 para amino phenol acetic anhydride reaction, 80â?? reactor temperature, 2 atm reactor pressure 60 min reactor residence time and 5 litres reactor volume gave the best optimum kinetic operating conditions. Moreover, the results show remarkable improvement in yield of acetaminophen; this showed that the modelling, simulation and sensitivity analysis is a promising method to improve complex processes.
Keywords
Kinetic modelling, Sensitivity analysis, Acetylation, Reactor, Acetaminophen, Aspen plus
Introduction
Increasingly as a result of better distribution channels, urbanisation in lower-middle income countries, and pandemic's long-lasting consequences. The formulation of Active Pharmaceutical Ingredients (APIs), the drug ingredients that result in the desired biological impact, is the primary focus of the upstream industry. Then, these goods are divided according to product segments [1]. The pharmaceutical industry has had the greatest growth in demand for analgesics during the past three years [2]. Both prescription and over-the-counter versions of these medications are available to relieve pain and reduce inflammation [3]. One of the most popular over-the-counter medications on the market, the API paracetamol, was the focus of this study. Acetaminophen has an anti-inflammatory action and is therefore prescribed for a broad range of chronic diseases and a myriad of clinical problems, which accounts for its high demand. Acetaminophen is produced as a powder [4]. Then, this powder is made into tablets, capsules, liquid solutions, or intravenous fluids. Around 80,000 metric tonnes of the bulk analgesics' paracetamol component are produced annually, with an estimated worth of $400 million. This estimate takes into account the global market availability price range of $3.5 to $5 [5].
The chemical molecule N-acetyle-P-aminophenol or 4-hydroxy acetanilide, with the molecular formula C8H9NO2, is more often known as paracetamol or acetaminophen. The melting point of this substance, which has a molecular weight of 151.2 and a white, crystalline powder, lies between 168 and 172°C. It dissolves completely in water (1-5 g/100 ml), but only slightly in ether or methylene chloride. It is soluble in alcohol. Likewise, paracetamol, also known as acetaminophen, is commonly used as analgesic and antipyretic drug and is produced more than 100,000 tons per year universally. Apart of being used directly, some scientists tried to improve paracetamol by modifying the molecules. Adi, et al. concluded that 3,5-dialkyl substitution resulted in lower chance of inducing hepatotoxicity [6]. It was also found that substituting acetic acid to the molecule can significantly enhance the antipyretic and analgesic effect. Paracetamol can be synthesized from different starting compounds. For instances, some scientists synthesized paracetamol from nitrobenzene by means of catalytic hydrogenation in the presence of acid some used p-nitrophenol as starting and some others studied the reaction pathway from hydroquinone [7-10]. Although the starting materials were different, many pathways led to the reaction of para-aminophenol acetylation with the addition of acetic anhydride to produce paracetamol.
Kinetic modelling and simulation is one of the most unique method of simulation that guarantee the actual behaviours of developed process subjected to research and development, this is due to the used of real experimental kinetic data in order to gain insight in a very difficult or rigors process design [11]. The used of kinetic simulation did not only relay on research data, it also helps in minimized the cost using real system with less time consumptions and gave almost 99% accuracy [12,13].
The use of computer simulation as an aid to learning is possible. It had been applied to the study of a variety of pharmaceutical-related problems, including pharmaceutical industry management, pharmaceutical formulations pharmacokinetics pharmacotherapy and pharmaceutical calculation [14-16].
Aspen plus is a very powerful computer simulation software with wide range of thermodynamic model calculator and a vast data bank that gave the user ability to model all types of chemical process. Its user friendly, quick convergence and short time simulation. This research work employed Aspen Plus version 11.0 to model and simulate the acetylation reactor of the continuous acetaminophen production process.
Materials and Methods
Materials
Table 1 presents the materials, tools and their purpose used in this research work.
| S. No. | Materials/tools | Purpose |
|---|---|---|
| 1 | Aspen Plus software version 11.0 | Perform modelling and simulation |
| 2 | Flow sheet from literature for the adopted synthesis method | To check out the design parameters |
| 3 | Data sheet from literature | For validation of the model |
| 4 | Computer (HP, 8GB RAM, 1TB ROM) | Aspen one will be installed and operated |
Table 1: Materials/tools used in this research.
Methods
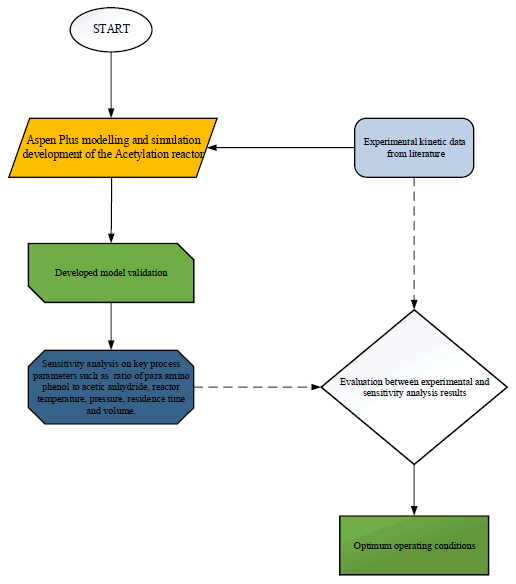
Figure 1 presented the schematic block flow diagram of the kinetic simulation method of acetylation reactor unit of the continuous synthesis of an Active Pharmaceutical Ingredient (API) acetaminophen.
Aspen plus simulation procedure: In this study, Aspen Plus was chosen as the platform for simulating the acetylation reaction unit. The basic procedures for running the simulation using Aspen Plus software are as follows:
- Lunch aspen plus
- Selecting the needed components
- Defining a fluid package
- Enter simulation environment
- Adding and specifying material streams
- Run simulation
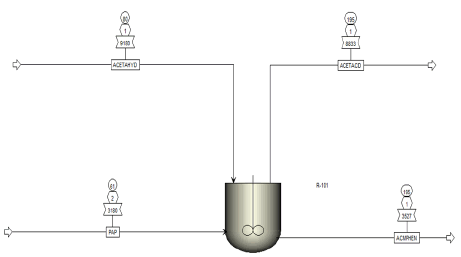
Process description: Figure 2 depicts the Acetylation reaction unit Aspen Plus process flow for the acetaminophen synthesis. The fed, para amino phenol and acetic anhydride entered the acetylation reactor, the p-amino-phenol was directed from the reduction unit, while acetic anhydride enter through the top of the reactor to produce acetic acid and acetaminophen as top and bottom product respectively. The Aspen Plus unit operation blocks utilised for the simulation are described in Table 2, and the feed composition entered for the simulation is shown in Table 3. The reactions that occur in each phase are summarised in Table 4, while the acetylation reactor specification presented in Table 5 respectively.
| Simulation name | Aspen Plus block | Uses |
|---|---|---|
| R-101 | RCSTR | To conduct acetylation reaction between P-amino phenol and acetic anhydride to produce API (Acetaminophen) and acetic acid |
Table 2: Process blocks description used in the simulation.
| Component | Temperature (°C) | Pressure (atm) | Flowrate (kg/h) |
|---|---|---|---|
| Para-Amino-Phenol | 61.07 | 1.5 | 3180 |
| Acetic anhydride | 80 | 1 | 9180 |
Table 3: Feed composition of the acetylation unit reactor.
| Reaction name | Equation for the reaction | K (S-1) | Ea (J/kmol) |
|---|---|---|---|
| Acetylation | 2pC6H7NO+C4H6O3?C8H9NO2+CH3COOH | 7.7E+09 | 143,857 |
Table 4: Chemical reactions involved in the acetylation reactor.
| Property | Value | Units |
|---|---|---|
| Volume | 20 | L |
| Residence time | 30 | min |
| Diameter | 0.6777 | mm |
| Height | 0.5 | mm |
| Material | Carbon steel | |
| Reactor type | CSTR |
Table 5: Reactor specification.
Assumptions for running the simulation: The following assumption were made for the running of simulation in Aspen Plus environment.
- Simulation is run at steady state
- CSTR is model as the acetylation reactor
- Reactor is assumed isolated from the atmosphere
- Catalyst not considered in the reactor
Model validation: Validation of the developed acetylation reaction unit was carried out using an existing experimental data, this to confirm that the model can be duplicated with the various process parameters and circumstances listed in Table 3-5 respectively.
Sensitivity analysis: When numerous orthogonal parameters may change simultaneously, complicating the analysis, sensitivity analyses (one component at a time) have the disadvantage of not correctly reproducing real-world conditions. The processes being evaluated are significantly impacted by important process factors that are related to the process design and model construction, yet they cannot be found via optimization techniques or conventional process analysis. The sensitivity analysis is performed using the Aspen plus version 11.0 case study analyzer, the parameters considered in running the sensitivity analysis are ratio of para amino phenol to acetic anhydride feed to the reactor as showed in Equation 1. Other factors considered are the effect of rector temperature, pressure, volume and residence time against the yield of acetaminophen.
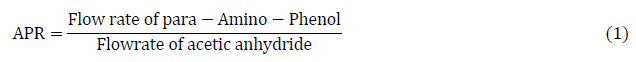
Results and Discussion
The validated acetylation unit of the continuous synthesis pathway process models for the active ingredient in paracetamol (acetaminophen) were simulated to conduct extensive sensitivity analysis on key process.
Developed aspen plus model validation
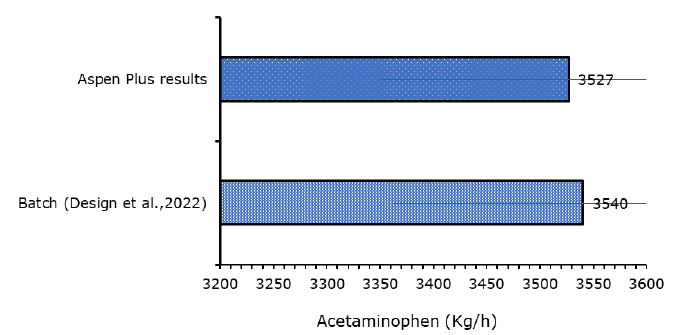
The acetylation unit reactor for the continuous acetaminophen synthesis pathway model must first be validated using the experimental study that was chosen from the literature, before the model's performance is examined. The experimental value of the chosen literature was compared to the production of the Active Paracetamol Ingredient (API), paracetamol. Figure 3 shows the outcomes of the model's comparison under the identical operating and process conditions.
The developed model was validated using experimental work of Riksen S, et al. comparing batch and semi-continuous pathways of acetaminophen production. Tables 2-4 provide the experimental operating condition which was used to run the developed Aspen Plus kinetic model. Moreover, from the results of validation (Figure 2); it was observed that the developed model gave the yield of acetaminophen as 3,257 kg/h at similar operating conditions, which is less than the experimental values 3540 kg/h. The experimental error between the result of literature and that of the Aspen Plus is (0.36%), which is less than unity and acceptable.
Sensitivity analysis
The sensitivity analysis of the key target component acetaminophen production process acetylation reaction unit was carried out using process kinetic data such as temperature, pressure, residence time and reactor volume.
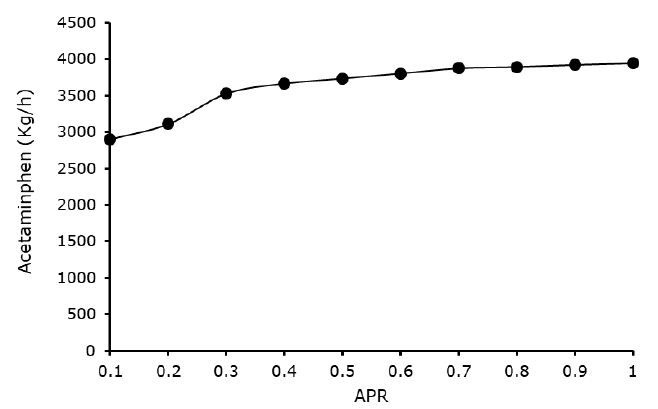
Effect of para-aminophenol acetic anhydride ratio: Figure 4 depicted the effect para amino phenol acetic anhydride ratio in the acetylation reactor, it was observed from the Figure 4 that the yield of acetaminophen rises steadily with the increased in para amino phenol acetic anhydride ratio from 0.1 to 1.0, with 1.0 with the maximum acetaminophen yield of 3,944.61 kg/h. This conclude that para amino phenol acetic anhydride ratio has significant effect on the acetylation reaction and the acetaminophen yield.
However, most of the work conducted by Ameen, et al. and Benista and Nowak. on continuous acetaminophen manufacturing route synthesis did not consider effect of the feed stock ratio, their emphasis relay on the temperature and pressure, this make their works suffer without determine the optimum acetaminophen yield.
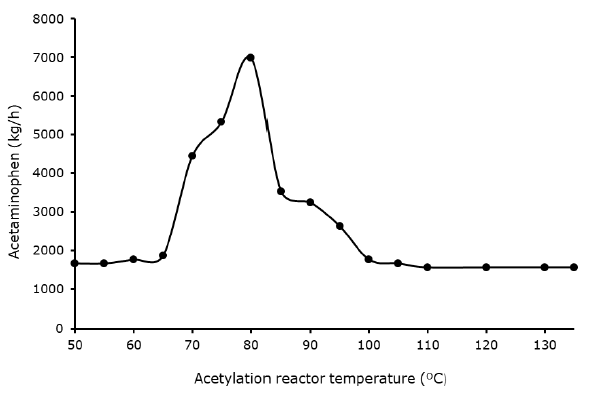
Effect acetylation reactor temperature against acetaminophen yield: The yield of acetaminophen was affected by temperature as shown in Figure 5. The acetaminophen yield remain steady from 50 to 65°C, the rises sharply from 70 to 80°C. It was revealed that with temperature of 80°C the maximum yield was achieved as (6,981.34 kg/h), subsequently declined steadily from 85°C respectively. The temperature with the optimum acetaminophen yield was almost similar to the results of the experiment as reported by.
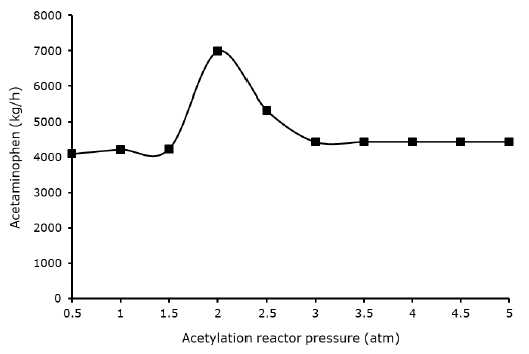
Effect acetylation reactor pressure against acetaminophen yield reactor: Pressure is another factor that affect the acetylation reaction continuous acetaminophen manufacturing pathway synthesis, it can be observe from Figure 6. the reactor pressure slightly affects the yield of acetaminophen, the reactor at pressure from 0.5 to 1.5 remain constant, while it rises at 2 atm, which gave the best optimum yield of acetaminophen (6981.38 kg/h), which 49.2% higher than the experimental results. The acetaminophen yield later decreases from 2.5 atm and remain constant from 3 atm above. This may be the rationale for why Riksen S, et al. thought that temperature in the acetylation reactor was more crucial than pressure.
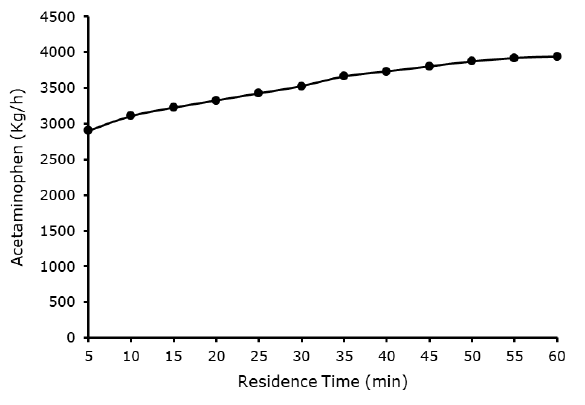
Effect acetylation reactor residence time acetaminophen yield: Figure 7 depicted in the effect of acetylation rector residence time against yield of acetaminophen. The residence time increases steadily, with increase in the yield of acetaminophen. However, it was observed from the Figure 7 that 60 min of the acetylation rector present the best optimum acetaminophen yield of 3944.61 kg/h. This can conclude that the higher the residence time the greater the acetaminophen yield.
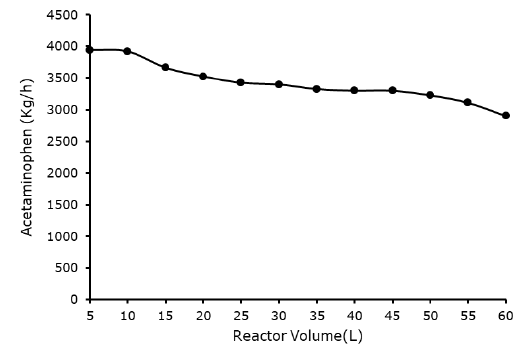
Effect acetylation reactor volume acetaminophen yield: The reactor volume is another variable that affected the yield of acetaminophen, which most the works conducted on the production acetaminophen neglected. The reactor volume significantly affects the yield of acetaminophen, the lower the reactor volume, the higher the acetaminophen yield, similarly the higher the volume the lower the acetaminophen yield. From Figure 8 it was observed that reactor volume of 5 liters gave the best acetaminophen yield of 3,944.61 kg/h, which is 10.25% higher that experimental work of Riksen S, et al. The results from the Figure 4 concluded that the lower the acetylation reactor volume, the higher the yield of acetaminophen produced.
Conclusion
The developed acetylation reactor kinetic simulation model using Aspen Plus under the continues acetaminophen synthesis was validated using UNIQUAC-Ideal as fluid package, the result of the validation was successful with 0.36% error, which is acceptable as it was less than unity. Also, the results of the sensitivity analysis revealed that 1.0 para amino phenol acetic anhydride and reactor temperature of 80°C, 2 atm reactor pressure, 60 min reactor residence time and 5 liters reactor volume gave the best optimum kinetic operating conditions. The sensitivity analysis of the acetylation reactor shows remarkable improvement of the acetaminophen yield from the previous studies. However, the optimum kinetic results can be used to improve the continues acetaminophen synthesis process.
Acknowledgements
The authors hereby extend their profound gratitude to the staffs of computer simulation laboratory, Chemical Engineering Department, Abubakar Tafawa Balewa University Bauchi.
Conflict of Interest
The authors declare that they have no conflict of interest.
References
- Riksen S, et al. ScholarlyCommons. 2022:4-18.
- Kazi MK, et al. Comp Chem Eng. 2021;145:107144.
- Sawalha R. Master's Thesis, Graz University of Technology. 2018.
- Shariare MH, et al. J Drug Deliv Sci Technol. 2018;43:122-128.
- Menacho WA, et al. Fuel. 2022;309:122058.
- Adi AH, Johari H, Sibai S. Int J Eng Bus Manag. 2020;4(3):51-56.
- Jolliffe HG, et al. Comp Chem Eng. 2018;118:224-235.
- Shafiq H, et al. Discov Chem Eng. 2021;1(4).
- Ahmad MA, et al. Pak J Sci Ind Res A: Phys Sci. 2022;65(3):302-308.
- Ottoboni S, et al. Powder Technol. 2020;366:305-323.
- Hidaka N, et al. Clin Ther. 2020;42(4):704-710.
[Crossref] [Google Scholar] [PubMed]
- Taipale-Kovalainen K, et al. Eur J Pharm Sci. 2018;115:1-10.
[Crossref] [Google Scholar] [PubMed]
- Sánchez-Paternina A, et al. Comp Chem Eng. 2019;124:109-123.
- Kekes T, et al. J Environ Chem Eng. 2020;8(6):104565.
- Cooke C, et al. Knee. 2020;27(6):1746-1752.
[Crossref] [Google Scholar] [PubMed]
- Cedron VP, et al. Biochem Pharmacol. 2020;174:113816.
[Crossref] [Google Scholar] [PubMed]