Reviews: 2022 Vol: 14 Issue: 6
Isolation and Characterization of Biological Active Compound from Root Extract of Thalictrum rhynchocarpum Dill
Fitsum Demissie1*, Legesse Adane2, Alemu Lelago2
1Department of Pharmacy, Institute of Health, Bule-Hora University, Oromia, Ethiopia
2Department of Chemistry, College of Natural and Computational Sciences, Hawassa University, Hawassa, Ethiopia
- Corresponding Author:
- Fitsum Demissie
Department of Pharmacy
Institute of Health,
Bule-Hora University,
Oromia, Ethiopia
Received:03-May-2022, Manuscript No. JOCPR-22-50835; Editor assigned: 6-May-2022, PreQC No. JOCPR-22-50835 (PQ); Reviewed: 21-May-2022, QC No. JOCPR-22-50835; Revised: 2-Jun-2022, Manuscript No. JOCPR-22-50835 (R); Published: 17-Jun-2022, DOI: 10.37532/0975-7384.2022.14(6).034.
Abstract
Background: Herbs/plants are among the major components of traditional medicine which have been used to tackle health care challenges by the vast majority of the world communities. In Ethiopia, Thalictrum rhynchocarpum Dill is one of the most commonly used plant species. Different parts of this plant species are traditionally used for the treatment of bacterial, fungal and malarial infections. Thus, investigating its chemical profile is crucial to explore bioactive constituents that could be used as lead compounds in drug discovery. Objectives: The objective of this study was to carry out phytochemical screening tests and isolate and characterization of compounds isolated from methanol:chloroform (1:1) extracts of root of Thalictrum rhynchocarpum. Method: The plant material was collected from the Keffa forest, Ethiopia, where diverse species of plants are available. The plant samples were transported to Jimma University laboratory of Organic Chemistry and dried under a shaded area for 15 days. The plant materials were pretreated and then were subjected to extraction with chloroform:methanol (1:1) using the maceration technique. The solvent was evaporated using Rota vapor and the residue was reconstituted and was subjected to screening tests and chromatographic separation. Results: The phytochemical screening results revealed the presence of flavonoids, alkaloids, phenol and terpenoids. 7, 3’, 4’-trimethoxy quercetin was isolated from methanol:chloroform (1:1) crude extract. Conclusion: Phytochemical screening test revealed the presence of secondary metabolites that could be attributed to the observed traditional medicinal use of this plant. Moreover, 7, 3’, 4’-trimethoxy quercetin (3,5dihydroxy- 7,3’, 4’-trimethoxy flavon) was isolated from this plant species.
Keywords
7,3’,4’-Trimethoxy quercetin; Secondary metabolites; Phytochemical screening; Extraction; Traditional medicine
Introduction
Traditional medicine is the combination of the practices, knowledge, skill, and based on the theories, beliefs, and indigenous experiences to different cultures, used in the maintenance, prevention, diagnosis, improvement, or therapeutic management of health problem1. According to WHO, more than 80% of the total population of developing country’s health care and wellbeing are solely depend on traditional medicine [1,2]. Medicinal plants are the major sources of ingredients (extracts) that have been and being used in traditional medicine in the world [3,4]. The medicinal uses of the plants have been attributed to the presence of secondary metabolites such as alkaloids, terpinoids, glycosided, phenols, anthraquinones [5, 6].
Similar to people elsewhere in the world, plant-based traditional medicine is used by majority (75%) of the people in in Ethiopia and the use traditional medicine in the health care system of the country is due to its availability, affordability and acceptability [7].
T. rhynchocarpum (locally known as Sire bezu) is one the several medicinal plants that are being used widely in traditional medicine in different parts of the world including Ethiopia [8,9]. For instance, it is used for blackleg disease by crushing a whole part of the plant 8. Its root is used for urinary tract infection by crushing and taking it orally [10,11]. The root part is also used to treat malaria, and leave as anti-insecticidal and to expel intestinal worms [12]. There are also reports that state the use of the plant to treat Donkey warts, and also to treat mumps, otorrhea, and ascariasis[13,14]. However, there are no reports on studies carried out on this plant to investigate its chemical constituents (secondary metabolites and bioactive compounds). Thus, the present study describes phytochemical screening testing of secondary metabolites present in the root extract of Thalictrum rhynchocarpum and also isolation and structural elucidation of biological active compound from the extract.
Materials and Methods
Plant Material Collection and Extraction
The plant materials (root parts) were collected from Keffa forest in the Southern Nations, Nationalities and Peoples Region (SNNPR), Southern Ethiopia. The area is located in the southwest direction from Jimma. The area has a latitude and longitude of 7°16′N 36°14′E with an elevation of 1,714 meters above sea level [15]. The plant was registered at The National Herbarium with voucher identification number YS001/2016.
The collected plant materials were transported to The Institute of Health, Jimma University. The plant materials were washed and allowed to dry in a shaded place for 15 days. The dried plant materials (root parts) were ground with a mortar and prepared for extraction. The ground materials were subjected extraction using a maceration technique [16]. The solvent system used for the extraction was a mixture of methanol and chloroform (1:1). The plant material (560 gram) was soaked in 4 Liter of solvent, and was put on shaker (GS-10/20/30) and was allowed to shake for 2 hours with occasional shaking. The mixture was filtered with cotton and filter paper consecutively. The filtrate was then concentrated under reduced pressure using Rota vapor (Labo rota 4000 Heidolph).
Purification of Crude Extract
The crude extract (65 gram) then subjected to chromatographic separation. 500 gram activated silica gel (60-80 mesh size) was used to pack the column. The silica gel was activated in the oven for 2 hours at a temperature of 120°C. The silica gel was made to slurry using HPLC grade n-hexane. The packed column was allowed to stay for 12 hours (overnight). The silica gel adsorbed crude extract was loaded slowly into the column packed with silica gel. A small amount of hygiene cotton was added next to the loaded sample to minimize the disturbance of the loaded sample during the elution of solvent [17, 18]. The elution of the column was begun with n-hexane. The polarity of the eluent was gradually increased from 99.5:0.5% (n-hexane:ethylacetate) until it reaches 75:25% (n-hexane:ethylacetate). Four collected fractions were monitored with TLC and visualization under UV-vis chamber (a wavelength of 254 and 344 nm) led to identification of a colored solid compounds. The compound was characterized based spectral data and comparison with literature reports. The NMR spectral analyses were carried out at The Department of Chemistry, Addis Ababa, Ethiopia.
Results and Discussion
As it has been stated in the introduction section, the plant used in this study is used for traditional medicine in treatment of several infectious diseases and as anti-insecticidal and to expel worms. Thus, in this study, investigation of the phytochemical constituents of the plant was carried out. The results revealed that the presence of flavonoids, alkaloids, phenol and terpenoids (Table 1). The finding is consistent with literature reports that state the presence of glycosides, alkaloids in the stem bark and root extracts, and flavonoids and triterpenes in the leaf extracts [19]. Also, this is in line with previous reports that state existence of secondary metabolites could be taken as supporting evidences for the observed traditional medicinal use of this plant for treatment of microbial infections [20].
Table 1: The result of secondary metabolites test for chloroform: methanol (1:1) crude extract
| Type of test | Strength |
|---|---|
| Alkaloids (Wagner’s reagent) | ++++++ |
| Flavonoids (Alkaline reagent test) | +++ |
| Phenols (Ferric chloride test) | +++++++++++ |
| Saponins (Foam test) | +++ |
| Sterols (Liebermann-Burchard test) | --- |
| Tannins (Braymer’s test) | ++++ |
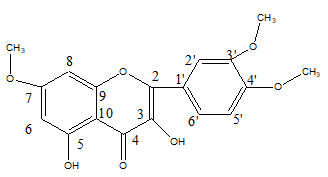
The crude extract was also subjected to chromatographic separation. This result in isolation of a crystalline solid compound (mp: 316.1 to 317.2°C). In the IR spectrum of the compound showed a broad band at 3400 cm-1 suggesting the presence of hydroxyl (OH) group, Moreover, a sharp and strong absorption band at 1700 cm-1 and a weak band at 1300 cm-1 could be attributed to carbonyl group and C-O-C group, respectively (Supplementary Material 1). The 1H-NMR spectrum (Supplementary material 2) showed two peaks at δ6.13 (1H, d, J=2.0 Hz) and 5.99 (1H, d, J=2.0 Hz) consistent with aromatic meta protons on A-ring and an ABX system at 6.96 (1H, d, J=2.2 Hz), 6.97 (1H, dd, J=2.0 Hz, 8.4 Hz), and 6.66 (1H, d, J=8.4 Hz) corresponding to the catechol protons on B-ring [19,20,21]. Furthermore, three peaks at δ 3.87, 3.66 and 3.64 could be attributed to three methoxy groups attached to aromatic rings. The two broad singlets at δ 8.23 and 13.0 could indicate the presence of two phenolic protons in the isolated compounds [22]. The spectral pattern was found to indicate the compound could be of flavonoid-type compound. The13C-NMR spectrum (Supplementary material 3) indicated the presence of fifteen carbon atoms, the signal at δ 198.3 was attributed to a ketone-type carbonyl carbon placed at C-4 of A-ring of flavonoid skeleton 20. In addition to this, three strong singlet peaks appeared at δ 55.1, 55.2 and 60.6 of the 13C-NMR spectrum which are characteristic signals for methoxy carbons. This observation is consistent with the 1H-NMR spectrum that revealed three methoxy protons. A thorough analysis of the spectral data and comparison with literature reports suggest that the compound isolated from this plant species to be 7,3’,4’-trimethoxy quercetin (3,5dihydroxy-7,3’,4’-trimethoxy flavon). The 1H-NMR and 13C-NMR data are consistent with the proposed structure (Table 2). In the 1H-1H–COSY (Supplementary material 4) spectra cross peaks were observed between the proton signals at δ 6.66 (H-2’) and 6.97(H-3’) and between proton signals at δ 6.13 (H-6) and 5.99 (H-8). These results were identical with the methoxy substituted quercetin 19. All the protons were assigned to their respective carbon atoms with the help of HSQC spectra (Supplementary material 5). The nine methoxy protons at δ 3.87 (3H, s, OCH3-7), 3.66 (3H, s, OCH3-5’) and 3.64 (3H, s, OCH3-4’) correlated with three aromatic carbons at δ 166.9 (C-7), 143.9 (C-5’) and 149.9 (C4’), respectively. In HMBC (Supplementary material 6), which determined the positions of these three methoxy groups. The phenolic proton at δ 13.0 correlated with three aromatic carbons at δ 163.2 (C-5), 106.3 (C-10) and 93.4(C-6) and suggested that the phenolic proton is present at C-5. Furthermore the following correlations were observed in the HMBC spectra, the proton at δ6.97 (H-2’) and 6.66(H-3’) are correlated with carbons at δ 149.9 (C-4’), 143.9(C-5’) and 137.7 (C-3), the proton at δ 5.99 (H-8) correlated with carbons at δ 93.4 (C-6), 163.2 (C-9) and 166.9 (C-7) and the proton at δ 6.13 (H-6) correlated with carbons at δ 90.8(C-8), 106.3(C-10), 166.93 (C-7) and 163.2 (C-5). The data are consistent with literature report for 3,5-dihydroxy-7,3’,4’-trimethoxy flavon isolated from Euodia confusa and fruit of Amomum koenigii (Figure 1) [23].
Table 2: The13C-NMR and 1H-NMR spectral data of the compound
| Position | Carbon signal (δ) | Proton signal (δ) | Position | Carbon signal (δ) | Proton signal (δ) |
|---|---|---|---|---|---|
| 2 | 167.3 | - | 1’ | 123.5 | - |
| 3 | 137.7 | - | 2’ | 117.5 | 6.97 |
| 4 | 196.2 | - | 3’ | 117.1 | 6.66 |
| 5 | 163.2 | - | 4’ | 149.9 | - |
| 6 | 93.4 | 6.13 | 5’ | 143.9 | - |
| 7 | 166.9 | - | 6’ | 123.5 | 6.96 |
| 8 | 90.8 | 5.99 | OCH3- 4’ | 55.2 | 3.64 |
| 9 | 163.2 | - | OCH3- 3’ | 60.6 | 3.66 |
| 10 | 106.3 | - | OCH3-7 | 55.1 | 3.87 |
- 1H-NMR (500 MHz, CD3COCD3): δ 6.13 (1H, d, J = 2.0 Hz, H-6), 5.99 (1H, d, J = 2.0 Hz, H-8), 6.96 (1H, d, J = 2.2 Hz, H-6’), 6.97 (1H, dd, J = 2.0 Hz, 8.4 Hz, H-2’), 66 (1H, d, J = 8.4 Hz, H-3’), 3.87 (3H, s, OCH3-7), 3.66 (3H, s, OCH3-5’) 3.64 (3H, s, OCH3-4’), 8.23 (-OH, C-3) and 13.0 (1H, -OH, C-5).
- 13C-NMR (125 MHz, CD3COCD3): δ 137.7 (C-3), 196.2 (C-4), 163.2(C-5), 93.4 (C-6), 166.93(C-7), 90.8(C-8), 163.2 (C-9) 3(C-10), 123.5(C-1’), 117.5(C-2’), 117.1(C-3’), 149.9 (C-4’), 143.9(C-5’), 123.5(C-6’), 55.2(OCH3- 4’), 60.6(OCH3- 5’), 55.1(OCH3-7)
Conclusion
In this study some secondary metabolites have been detected from the from the methanol and chloroform extract of the root of the plant used in the study. Moreover, 7, 3’, 4’-trimethoxy quercetin was isolated from the root extract. The structure of the compound was elucidated based on the spectroscopic data and comparison with literature reported data. It is recommended to carry out extraction with different solvent systems in order to exhaustively investigate the phytochemical constituents and compounds. Biological activity tests are also recommended on both crude extracts and isolated compounds in search of candidate compounds for discovery of new drugs from this plant.
References
- Zamawe C, King C, Jennings HM, et al.BMJ open. 2018;8(10):022499
- Tauchen J, Doskocil I, Caffi C, et al.IndustriCropProduct. 2015;74:671-679.
- Erdemgil FZ, Ba?er KH, Kirimer NE. ACTA PharmaceutSci. 2001;43:3-4.
- Gurunathan AB, Subramanium PA, Maran SA. Int J Pharm Pharm Sci. 2013;5:71-5.
- MayekuPW,OdaloJO,HassanaliA,etal.JChemPharmRes.2014;6(11):1?5.
- Wink M. Med.2015;2(3):251-286.
- KassayeKD,AmberbirA,GetachewB,etal.EthiopianJHealthDevelop.2006;20(2):127.
- MegersaM,AsfawZ,KelbessaE,etal.JEthnobioEthnomed.2013;9:1?8.
- MayekuaPW,HassanalibA,KiremireaBT,etal.AfricaJTraditionComplementAlternatMed.2013;10(5):341-344.
- GidayM,AsfawZ,WolduZ.JEthnopharmacol.2010;132(1):75-85.
- ChekoleG,AsfawZ,KelbessaE.JEthnobioEthnomed.2015;11(1):1?38.
- Yineger H, Kelbessa E, Bekele T, et al. JMedPlantRes.2013;2(6):132-153.
- Birhan YS, Kitaw SL, Alemayehu YA, et al. SM JMedPlant Stud. 2017;1(1):1-9.
- Lulekal E, Rondevaldova J, Bernaskova E, et al. Pharm Biol. 2014;52(5):614-620.
- Woldegebriel AA, Amibo TA, Bayu AB. Sri JEnvironment. 2021;6(2):36-52.
- Sasidharan S, Chen Y, Saravanan D, et al. African JTradComplementAltMed: AJTCAM.2011;8(1):1-10.
- Kebede T, Gadisa E, Tufa A. PLoS One.2021;16(3):e0249253.
- Beller NR, Hilleary CJ. JChemEdu.1976;53(8):498.
- Versiani MA, Diyabalanage T, Ratnayake R, et al. JNatProducts. 2011;74(2):262-266.
- Cuong DTD, Dat HT, Duan NT, et al.VietnamJChem.2019;57(4):438-442.
- Higa M, Imamura M, Ogihara K,et al. ChemPharmaceut Bull. 2013;61(4):384-389.
- Blunder M, Orthaber A, Bauer R,et al. Food Chem. 2016;09(01):218
- PhanMG,DoTVH,NguyenQB.BiochemResInt.2020;53(8):498.