Original Articles: 2023 Vol: 15 Issue: 7
Industrial Wastewater Colour and Toxic Heavy Metals Removal by Biosorption-Using Marine Algae and Moringa Olifera Seed
Samuel Paul Prasanna T*, Supriya Subrahmanian
Department of Chemistry, Sathyabama Institute of Science and Technology, Tamil Nadu, India
- Corresponding Author:
- Samuel Paul Prasanna T
Department of Chemistry,
Sathyabama Institute of Science and Technology,
Tamil Nadu,
India
Received: 30-Dec-2019, Manuscript No. JOCPR-23-5991; Editor assigned: 02-Jan-2020, PreQC No. JOCPR-23- 5991 (PQ); Reviewed: 16-Jan-2020, QC No. JOCPR-23-5991; Revised: 30-Jun-2023, Manuscript No. JOCPR-23- 5991 (R); Published: 28-Jul-2023
Citation:Prasanna SPT, et al. 2023. Industrial Wastewater Colour and Toxic Heavy Metals Removal by Biosorption-Using Marine Algae and Moringa Olifera Seed. J. Chem Pharm. Res., 15:037.
Copyright: © 2023 Prasanna SPT, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
With rapid modern industrialization there is an increases of the growth rate of various pollution sources in the universe. Industries releases wide range of dissolved heavy metals as inorganic and phenolic compounds as organic pollutants in an effluent. Industrial effluents having heavy metals were known as a major important resource for water pollution. The contaminations make large volume of environmental crisis that are being faced by the humanity today. Various treatment processes are available and are extensively studied for the treatment of the polluted waters and wastewaters from various industrial sectors. One of the most promising and viable methods for wastewater treatment is the biosorption method. Biosorption is used as an alternate treatment method and are replacing the conventional methods. Moringa olifera (activated carbon) and marine algae are used in the biosorption process of treatment of tannery effluent water. Marine algae adsorb large amount of heavy metallic ions is due to the availability of different chemical functional groups on the cell surface, as a result of physico-chemical interaction. In this study, biosorption of Cr (VI) and Pb (II) ions from aqueous solutions using Moringa olifera and Marine algae (Kappaphycus alvarezii) is investigated as a function of pH, initial metal ion concentration, biosorbent dose and contact time. At the optimum adsorption pH values, the maximum uptake capacity for chromium is estimated as 73.106 mg g-1 and for lead 79.145 mg g-1 . Equilibrium data is well described by the Langmuir and Freundlich adsorption relations. The presence of functional groups is confirmed using (FTIR) spectroscopy and the metal ions are analyzed by AAS method using specific lamps at specific wavelength. The result shows that Moringa olifera (activated carbon) and marine algae as biosorption materials has very good potential for the removal of heavy metal ions and colour from the tannery wastewater.
Keywords
AAS, Biosorption, Chromium, FTIR, Isotherms, Lead, Marine algae (Kappaphycus alvarezii), Moringa oleifera, Tannery effluent
Introduction
Industrial heavy metallic ion wastes are often produced from various industrial activities. As a result, the amount and toxicity of heavy metal waste released from industrial activities varies with the industrial processes. A heavy metal is a collective term for metals of high atomic mass, particularly those that are toxic and cannot be processed by living organisms [1].
These include lead, chromium and cadmium among others. Depending on the context, the term can include elements lighter than carbon and can exclude some of the heaviest metals. Heavy metals can be broadly classified into the following groups such as those that are essential for certain biochemical processes, but are toxic when their concentration exceeds certain thresholds and those that are the most dangerous group which includes lead, chromium, cadmium and mercury which serves no known biological function and are toxic at all concentrations [2].
The common results of trace metal toxicity to living organisms includes brain disorder, gross deformities in development, carcinogenic effects and generally, disruption of biological processes. In most cases these elements find their way into the environment through human industrial processes such as mining, electroplating, battery manufacture, leather tanning and manufacture of printing pigments and paints, among others. A high concentration of heavy metals in the environment is of great health concern because they are non-biodegradable and end up accumulating in food chains in various forms such as organic, inorganic or organometallic species. Due to the toxicity of trace metals, it is important to remove them from water in particular and the environment in general [3].
MATERIALS AND METHODS
Collection of sample
Tannery industry wastewater was collected from Ms.Sara leathers industries Pvt Ltd, Ranipet, TN, India and was stored in refrigerator to prevent further contamination. The Moringa olifera which are grows abduntaly in Karunguzi village near Villupuram district, TN, was collected and kept separately. The matured Moringa olifera was dried under sunlight for one week. After drying, the Moringa olifera inside seed pods (husk) was made into activated charcoal by several treatment processes and was stored. The activated carbon consists 89%-98% as carbon and the other elements viz. N, O, S and H. The studies shows that the adsorption capacity of the commercially available activated carbon is closure to that of the activated carbon prepared from Moringa olifera [4].
Preparation of biosorbent (macro algal biomass)
Species of macroalgae Kappaphycus alvarezii a species of red algae which is vailable abundantly in the coasts of Tuticorin, Tamil Nadu is taken and it washed with millipore water and it is kept in shade dried and it is taken in an oven for drying at 60°C overnight. The dried algae were then grounded into particle size of 0.5 milli meter. Prepared algae biomass were kept preserved in a polythene cover bags for the biosorption experiments to be carried out [5].
Preparation of synthetic metal solutions
Potassium dichromate reference material was used for the preparation of synthetic chromium (VI) solution. A stock solution of chromium VI (Cr+6) was developed by adding potassium dichromate (analytical reagent grade) in 1 L of 0.1 N NaOH solution. Take from a prepared stock solution of 100 ppm/L, different concentrations like 2.0 ppm, 4.0 ppm, 6.0 ppm, 8.0 ppm and 10.0 ppm were prepared using millipore water [6].
Biosorption studies in batch process
Tannery industry wastewater was passed through the activated carbon of, which were soaked in ethanol for 48 hrs and were filtered out using whatman No.1 filter paper, the tannery wastewater colour was removed to the maximum along with the removal of chromium and lead. The filtrate wastewater, after colour removal, was used for biosorption studies. The biosorption studies were conducted using 100 ml volume of chromium and lead contaminated tannery aqueous solution in 500 ml Erlenmeyer flasks. Impact of parameters such as pH (1, 3, 5, 7 and 9), contact time (15, 30, 45, 60, 90 and 120 min), heavy metal ions concentrations (1 mg/kg, 3 mg/kg, 5 mg/kg, 7 mg/kg and 9 mg/kg) and red marine algal biomass (1.0 g/100 ml, 2.0 g/100 ml, 3.0 g/100 ml) on heavy metal ion biosorption was studied by varying the parameters at a time and taking all the other parameters constant [7]. Biosorption process was conducted at the temperature of room temperature. The first monolayer Metal Ion Concentration (MCi) in the liquid solution was calculated according to APHA, 23rd edn. 2017 using agilent make Atomic Absorption Spectrophotometer (AAS). Mixtures were mixed step by step in a rotary shaker machine by fixing the agitation rate between 100 rpm to 150 rpm for 60 min. The biomass solid phase was filtered off using grade 1 No.1 ash less filter paper after kept settling for 60 mins. Heavy metallic ion concentration in the filtrates (MCf) was studied using AAS [8].
Calculation of metal ions
Experiments were carried out in duplicates and the results were averaged out for the analysis for biosorption. The percentage of heavy metals (lead and chromium ions) was given as follows for biosorption.
Biosorption (%)=(MCi-MCf )/MCi × 100 (Pb and Cr)
where MCi is the first metal ion concentration (initial) of lead (mg/l) and chromium (mg/l) and Cf is the end metal ion concentration (final) (mg/l).
Isotherms studies
Isotherms can be studied by varying the first concentration of the metallic ions at the available conditions for every metal. Various biosorption tested models were taken for differentiating of experimental values. SEM having very high resolutions and gives larger magnification for the closely spaced available elements. In the modern stage studies, the monolayer morphology for the controlled (pre-treated), the biosorbed red marine algae was studied using SEM (Scanning Electron Microscope) [9].
RESULTS AND DISCUSSION
The red marine algae was used for biosorption of lead (Pb) and chromium (Cr) of liquid solution and the values of contact impact of each test parameter are given below.
Contact impact of pH
The contact impact of pH for the metallic ions biosorption of chromium (Cr (VI)) and lead (Pb (II)) on to the macroalgal biomass was evaluated. It was observed that the biosorption capacity was increasing with the increase of pH from 2 to 10. The pH is considered to be the most important parameter which plays a major role in the biosorption of metal ions from solutions. At lower pHs, the active sites on the biomass surface would be occupied mainly by the protons due to its high concentration [10]. This would restrict the attachement of the metallic cations towards the monolayer of the red marine algae biomass. As the pH increases, with a decrease in the proton concentration, the biomass surface becomes more negatively charged and enhances the biosorption of the positively charged metal ion attaining maximum biosorption in the pH range of 5-7 for Kappaphycus alvarezii. According to our findings, the maximum uptake of chromium (VI) and lead (II) by the red algae was at pH 5.09 and 6.24 respectively as given in Table 1. The increased biosorption with increasing pH values can be attributed to the presence of acidic CO and RSH groups of cell walls of the red marine algae biomass and for the metallic ion studies [11].
| S.No | Metal ions with biosorption system | Biosorption max. at different pH values | ||||
|---|---|---|---|---|---|---|
| 2 | 3 | 4 to 5.5 | 5.5-7.0 | 7 to 10 | ||
| 1 | Lead as Pb (II) | - | - | - | 6.24 | - |
| 2 | Chromium as Cr (VI) | - | - | 5.09 | - | - |
Table 1: Study of the influence of pH for biosorption in metallic ions.
Influence of biosorbent dosage
The behavior of the marine red algae biomass addition on the biosorption process of chromium (VI) and lead (II) from solution shows a steep increase in the chromium (VI) and lead (II) uptake by the biomass of Kappaphycus alvarezii upon increasing the dosage from 0.60 g/100ml to 3 g/100 ml [12]. Red marine algae biomass dosage of 3 g/100 ml had the maximum removal of 92% and 76% for chromium (VI) and lead (Pb (II)) respectively from the aqueous solution as given in Table 2. The percentage uptake of chromium (VI) and lead (II) was found to be gradually decreasing above the optimum biosorbent dosage. This effect could be due to the formation of aggregates of the biomass at higher dosages which in turn decreases the surface area available for surface adsorption of metal ions [13].
| S.No | Biosorption system with metal ions | Maximum removal of metal ions using dosages from 0.6 g to 3 g/100 ml |
|---|---|---|
| 1 | Lead as Pb (II) | 92 g/100 ml (%) |
| 2 | Chromium as Cr (VI) | 76 g/100 ml (%) |
Table 2: Study of the effect of biosorbent dosage on metal biosorption.
Effect on contact time
The way of contact time was noted to large influence in the biosorption contact process which is indicated from the effect on the biosorption of chromium (VI) ions and lead (II) ions using an optimum biomass (3 g/100 ml) of Kappaphycus alvarezii. The results showed that the biosorption of chromium (VI) and lead (II) was rapid during the first 30 min and then showed a gradual increase with the attainment of equilibrium at 90 min. The uptake capacity did not show any improvement after the contact time of 90 min as shown in Table 3 [14].
| Concentration (g/100 ml) | Lead as Pb (II) and Chromium as Cr (VI) uptake | ||
|---|---|---|---|
| 30-60 mins | 60-90 mins | 90-120 mins | |
| Biomass (3 g/100 ml) | - | 89% Pb (II) | No change |
| - | 87% (Cr (VI)) | No change | |
Table 3: Study of the effect of contact time on metal biosorption.
Influence of metal ion concentration
The study of the effect of metal ion concentrations on the biosorption process showed that the biosorption of lead (II) and chromium (VI) was increasing by the increase 2.0 mg/kg to 10.0 mg/kg in the presence of the red marine algae. The maximum removal percentage was 71.45% and 77.1% in order at 10 mg/kg concentration of (Cr (VI)) and Pb (II) respectively as given in Table 4. The results indicate that the biosorption process was favourable even for high starting stages of metal ions concentration. This within the agreement with the previous report on the studies of the biosorption process of the heavy metal ions of lead from liquid solution by the non-living red marine algae biomass of Kappaphycus alvarezii sp. Biosorption was found to decrease with the increase in the metal ion concentration above 10 ppm. The increase is due to the presence of large number of vacant binding sites initially leading to higher and faster biosorption and at the later stage the decrease is due to the availability of less number of active sites on the surface of the algal biomass [15].
| Metal ions concentration (mg/L) | Metal ion removal (%) lead as Pb (II) and chromium as Cr (VI) |
|---|---|
| 2 to 10 mg/L | 73.1 g/100 ml (%) of lead (Pb (II)) |
| 79.1 g/100 ml (%) chromium (Cr (VI)) |
Table 4: Study of the effect of metal ion concentrations on the biosorption of metal ions.
Adsorption isotherms studies
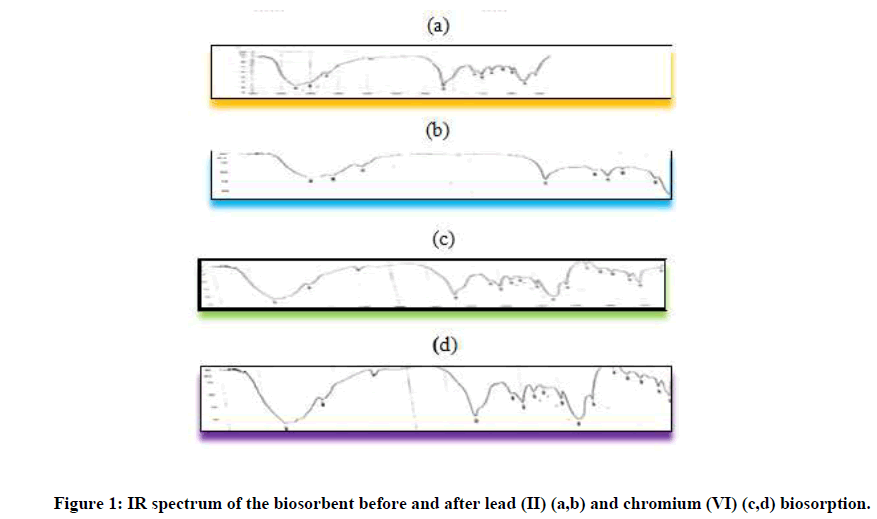
Langmuir and Freundlich isotherms studies shows adsorption isotherm phenomenon for the solid-liquid meeting. The value used for the modelling of the adsorption process and also to knows the relationship of the adsorbent and the adsorbate surface. The results of the Langmuir model parameters for chromium and lead biosorption by macroalgae showed that the efficiency of adsorption was minimum (qmax=1.41 ± 0.18 (Cr+6), 1.37 ± 0.11 (Pb+2)) with a high correlation coefficient (2.69 ± 0.31 (Cr+6), 2.33 ± 0.13 (Pb+2)) as given in Tables 5 and 6. These shows that single monolayer metal adsorption as well as heterogeneous metal surface conditions could complies under the studied situation. Hence Langmuir isotherm can be refere to model of the experimental metal adsorption data for the selected marine macroalgae data (Figure 1).
| Algae | qmax (millimol g-1) | b (L milli00 mol-1) | r2 |
|---|---|---|---|
| Kappaphycus alvarezii | 1.41 ± 0.18 (Cr+6) | 2.69 ± 0.31 (Cr+6) | 0.97 (Cr+6) and (Pb+2) |
| 1.37 ± 0.11 (Pb+2) | 2.33 ± 0.13 (Pb+2) |
Table 5: Chromium and lead adsorption isotherm model studies of Langmuir of marine algae.
| Before Cr (VI) and Pb ions biosorption | After Cr (VI) and Pb ions biosorption | Difference | ||
|---|---|---|---|---|
| Wave number (cm-1) | Annotations | Wave number (cm-1) | Annotations | |
| 3435 | O-H group | 3438 | O-H group | 3 |
| 2925 | CH2 group | 2923 | C-H stretching vibration | -2 |
| 2524 | S-H stretching vibration | 2525 | S-H stretching vibration | 1 |
| 1807 | Carbonyl group (C=O) stretching vibration | 1802 | C=C bond stretching vibration | -5 |
| 1624 | C=O stretching vibration of the ketone | 1624 | C=O stretching vibration of the ketone | 0 |
| 1471 | Vibration of the CH2 group | 1419 | Carboxyl COO- units | -52 |
| 1417 | Carbonyl group, C=O | 1036 | C-O and C-O-C stretching vibrations | -381 |
| 1082 | PO2-vibrations of phospholipids | 876 | C=O stretching vibration | -206 |
| 875 | CO3 vibrations of calcite | 719 | C-H bend of alkene | -156 |
| 716 | C-O-C bending vibration | 546 | Vibration of P=O in PO43- | -170 |
Table 6: FTIR values of Cr (VI) and Pb (II) of biosorption.
Fourier Transform Infrared (FTIR) spectroscopy was used for the analysis of the pre-treated and the biosorbed algal biomass samples. The algal biomass samples were made into a pellet using KBr and the IR spectral datas was gathered using an agilent make spectrophotometer instrument between the range 410 cm-1-4000 cm-1. The FTIR spectra of the samples are illustrated in Figure 1 for the unloaded biomass (control) and Chromium (Cr) and Lead (Pb) loaded biomass. The IR peaks correspond to the functional groups present on the surface of the cell wall of the biomass and their interaction with the metal ions. It is clear from Figure 1 that the FTIR spectra of the chromium and lead loaded Kappaphycus alvarezii biomass, showed a significant reduction in the peak intensity corresponding to (-OH) stretching bands (3401 cm-1 to 3435 cm-1). The decrease in the distance between the bands for the loaded biomass after metallic binding is by the attainment of large symmetry present in the cell wall as a result of metal-metal complexation formed. Similar type of interaction was also reported by the studies on the mechanism of adsorption of heavy metals such as chromium and lead by Kappaphycus alvarezii biomass. The comparison of the IR spectra of the unloaded red algae biomass of the metal ions loaded biomass shows the presence of functional groups such as NH (amino), carboxyl (COO), hydroxyl (OH) and carbonyl groups (CO) on the surface of the biomass cells and their interaction with the metallic ions. The difference in the band structure can be attributed to the nitrogen-hydrogen stretching band. Similarly, a significant shift is observed in the wave number from 2925 cm-1 to 2923 cm-1, corresponding to the C-H stretching band indicating the partcipitating of the C-H (methyl) stretching band to be in the binding of the chromium onto the macroalgae. Disappearance of the carbonyl group peak at 1082 cm-1 in the lead loaded biomass samples shows the effective interaction of the carbonyl group with chromium than with lead. For lead treated biomass sample, the involvement of the other functional groups involved in binding with the heavy metal is given in Table 6.
Scanning Electron Microscope analysis (SEM)
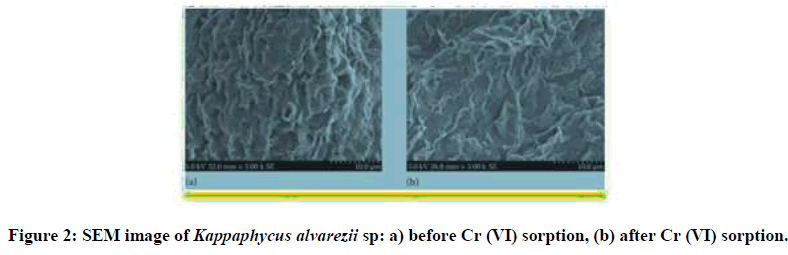
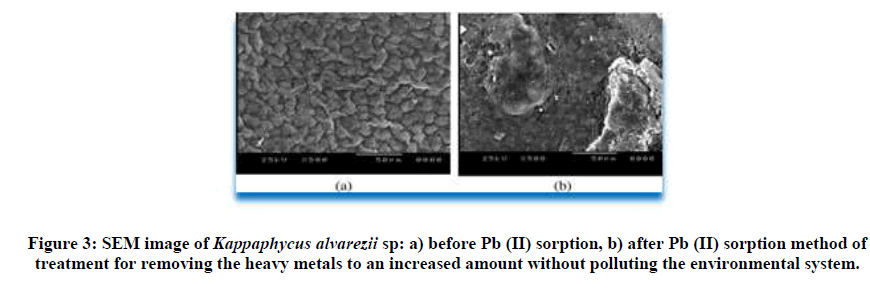
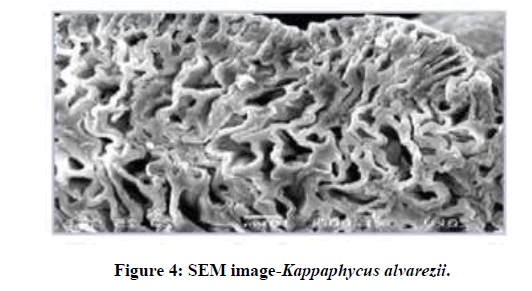
The outer surfacial morphological contol (unloaded biomass) and the biosorbed marine type algae were recorded using scanning electron microscope. The SEM image of the chromium and lead loaded red algae are shown in Figure 1 and Figure 2 respectively. Protuberances and microstructures observed on the surface of the unloaded Kappaphycus alvarezii biomass sample might be the presence of the calcium and the salt type crystal deposition as also observed at SEM analysis in raw Kappaphycus alvarezii sp. shown in Figure 3. The raw Kappaphycus alvarezii sp (red algae) was shown in Figures 2-5.
CONCLUSION
Biosorption and biomonitoring experimental studies conducted in this present work shows significant information regarding the suitability of red marine algae as a biosorbent material and a bioindicator for the identified heavy metal pollution. Adsorption parameters well determined. The optimum pH was found to be 5.8 and 5.0 for chromium and lead, respectively and the contact times required for the quantitative metal uptake were 40 min for Cr and 50 min for Pb. The adsorption capacities were found to be 71.106 mg/g and 77.145 mg/g for chromium and lead, respectively. The initial metal concentrations which resulted in highest metal adsorption onto red algae were between 300 mg/l and 600 mg/l for the two metals considered. The colour removal percentage was found to be 97% by using the activated charcoal obtained from Moringa olifera which acted as a good reducing agent in the tannery industry wastewater. As the availability of this plant in India is enormous like red algae it could be used for treating the wastewater as is highly economical.
From this work, red algae, Moringa olifera (as activated carbon) was found to be a good biosorbent which can be used for the effective removal of heavy metals and colour from tannery wastewater. The algae serves as a suitable bioindicator because of its ability to accumulate metals to a reasonable level. While the metal concentration in the water samples was negligible for the metals considered, the algae were much richer in heavy metal content. Due to the removal of heavy metals from polluted water by the biosorbents, determination of heavy metal pollution in any water body by direct analysis of water samples may not be accurate as it does not reflect the real bioavailable pollutant level in the water body. This is also because, most of the heavy metals will be removed from the water by the biota and the sediment resident in the same water. Use of a bioindicator like red algae would serve the purpose in a better way. Furthermore, work may be carried to find the efficacy in reduction of concentration of other heavy metals present in the tannery wastewater. New modern techniques may be adopted to increase the utility of this method of treatment for removing the heavy metals to an increased amount without polluting the environmental system.
References
- Chervona Y, et al. Metallomic. 2012;4(7):619-627.
[Crossref] [Google Scholar] [PubMed]
- Kumar JN, et al. J Environ Biol. 2012;33(1):27.
[Google Scholar] [PubMed]
- Davis TA, et al. Water Res. 2003;37(18):4311-4330.
[Crossref] [Google Scholar] [PubMed]
- Volesky B, et al. Biotechnol Progress. 1995;11(3):235-250.
[Crossref] [Google Scholar] [PubMed]
- Tobin JM, et al. Water Res. 1998;32(5):1407-1416.
- Das N, et al. Ind J Biotechnol. 2008;1:159-169.
- Srivastava NK, et al. J Hazard Mater. 2008;151(1):1-8.
[Crossref] [Google Scholar] [PubMed]
- Bailey SE, et al. Water Res. 1999;33(11):2469-2479.
- Khraisheh MA, et al. Chem Eng J. 2004;99(2):177-184.
- Mohammadi T, et al. Separat Purifi Technol. 2005;41(1):73-82.
- Pansini M, et al. Desalination. 1991;83(1-3):145-157.
- Perez-Candela M, et al. Water Res. 1995;29(9):2174-2180.
- Rengaraj S, et al. J Hazard Mater. 2001;87(1-3):273-287.
[Crossref] [Google Scholar] [PubMed]
- Mandi L, et al. Water Res. 1996;30(9):2009-2016.