Original Articles: 2023 Vol: 15 Issue: 11
Identification, Isolation, Structural Characterisation and Assessment of Toxicological Effect of Photo-initiator (2-Hydroxy-2-methyl-1- phenylpropane-1-one) in Ophthalmic Drug Product
Sandeep Zokande*, Kavita Inamdar, Pramod Borade, Vikas Mane
Indoco Remedies Limited, Navi Mumbai, India
Received: 24-Oct-2023, Manuscript No. JOCPR-23-118176; Editor assigned: 27-Oct-2023, PreQC No. JOCPR- 23-118176 (PQ); Reviewed: 10-Nov-2023, QC No. JOCPR-23-118176; Revised: 17-Nov-2023, Manuscript No. JOCPR-23-118176 (R); Published: 24-Nov-2023, DOI:10.37532/0975-7384.2023.15(11).061.
Citation:Zokande S, et al. 2023. Identification, Isolation, Structural Characterisation and Assessment of Toxicological Effect of Photo-initiator (2-Hydroxy-2-methyl-1-phenylpropane-1-
one) in Ophthalmic Drug Product. J. Chem. Pharm. Res. 15:061.
Copyright: © 2023 Zokande S et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Estimation of related substances is essential quality attribute to ascertain the quality of drug product during evaluation of shelf life and storage statement. Generally, related substances remain either stable or increases on time due to various environmental influences. Those substances may absorb on immediate Container Closure System (CCS) or decompose and converts into another compound having different toxicity/therapeutic benefits. Developing control procedure of related substances sometimes becomes difficult task due to their poor chromophore, solubility, and stability in drug product and in analytical solvents. Due to complex composition of CCS, regulators have introduced guidance for drug product manufacturer to incorporate leachable in drug product specification. Syntheticroute of drug substance and information about excipients, forced degradation, stress study, drug-excipient compatibilityprovide information about product related impurities; however, these studies does not provide details about leachable. Hence, understanding leachable is critical element during product development. Extractable and leachable studies may provide limited information due to typical lot used during study and techniques used. Even though the primary and secondary CCS are analyzed as per set standards, the control of leachable cannot be ascertained without prior information. Due to slow migration property, extractable study may give false or limited information. The componentsof CCS leach into the drug product and pose serious health hazards. Hence, it is extremely important to identify suchcompounds so that they can be adequately quantified and evaluated for toxicological/safety assessment.
Apart from leachable of immediate CCS, secondary packaging components such as label, ink and carton should also be evaluated for anticipated leachable. In this invention, an unknown leachable in ophthalmic solution is selected for identification, structural characterization, and toxicity assessment. The leachable finally characterized as 2-Hydroxy- 2-methyl-1-phenylpropane-1-onel.
icsatc neodeme advancedstructurescorp floormachinebrush scpe jcamasonry sages-tunisie sbsteel technomailleplus bolkan vaalea nsblueprinting mycleanairdoctors nycgeneralproroofing gabgadgets prsnekkern uniquescaffoldingsystems villaguicciardini acerpackaging acjstucco prontointervento-multiservice printersupplygiant sarel takkatiimi dragonshollow mazelsupply alfalchetto reliablegeneralagency lckenterprises realizzazione-giardini dmcindustries shop chathambrass ilfaroservizi soilmechanicsdrilling colorfullyyours agcsound carriere carnavaldetournai falconauto codingxcamp davinci-trondheim thebestofcolumbia cooperativetissage outsourcedmarketingpros tlc dawnnhough doubleclick pdirealty hattrennet az-bizsolutions jamaistropdart frontdata unitycreations fortisarezzo bspo-ken codar-confection theboulders bakerpersonnel fantasyphotographyandvideo fuleky tanssikoulutria ladybi swimanddance scuolavelatoscana x-pack creativeglazing lucagnizio sigaretteelettronichepisa mayiindustries frigo-clim elgars leoemma hotelalcantara el-pro therefore thebestofjacksonville nykran masmoudi thebestofspokane cogepre detrecruitment laresidencelepartage chimneycompanyboston awarepedia res-botanica recipefy semap thebestofcincinnati bb-one fourtech thebestofjoliet ajproduce jerryshulmanproduceshipper lckcabinetryny drivingtechniquesmadeeasy accademiaestetica pizzamaison schoonheidssalonbianca cabinsbrevardnc mrbrushes atlasrolloff hatip-medikal vezcocorporation thebestofcoloradosprings filtaclean monteleeper flyfisher ellatafa associazionepugliesiapisa unitycreationsltd maketeamstudiot douglasclear packagingpro softm allstarbeerinc burtonsupply footballscouting profondacreation johnvravickddsms fireislandbuilder advancedcontrolsolutions chittorgarhtaxiservices live-now orologeriatoscana racinemode thebestofwilmington agro-services violiner sandinghouse the-complete-package thebestofrichmond rcollision meubleskarray mes-recettes lawsandtaxes tavgrupp mkproducts royscottmarine taxi contreras-stockman sigmaweb de-noord thebestofkansascity maisonmedicaledelaeken recycledrubberpavers surfacingsystems unitedfidelityinc enokplan hunterremodeling edmersupply amerequipint sanilabcorp igglesis christiannursingregistry united-royal cantodelfiume northsidedeliny menuiserie-delbart passeritartufi justmyvoice ablefiresprinklers accantoalcentro ckperformance khalfallahpneus anti-flood-barriers bmstyle autosangiorgio-mercedes-benz carmagnino viipurinurheilijat multipack cma-eng mcafeerealty adrianaperciballi loconteedilecostruzioni konarprecision unityrubberco unityrubberproducts dante tela marathongranite baltimahalworcester heimdalbygg alstateprocessservice italydreamtour wemcocastingllc yachtbritesigns ecoledecroly-renaix anchorseniorapartments royalburton sk-veilag giannacapoti hubiteg customcommercialconstruction edm-nivelles probat-tunisie bachlawyer robertex inbora gpbconstruction tekstschrijver-tim bdsit trepro himalayanlounge lazersharpplumbing nycollisionking marinanova gealcorp nygabe maritimecoverage justmyvoice jukumech quimicolsa hayat-med aldrovandiauto michaelbenaltinc korenas sandvet americanmufflerautorepair justmyvoice liacoustics dminteriors agenziapromotech ligbtour internormfirenze justmyvoice zouila kohalmiferenc ashgroveresort ilmacinapepe suzukibandit hihna islandfishli prontointerventofabbro24h thebestofmidland thebestofportland dsgnaturaeambiente rimpex-medical giadaguidi royalroseappliances normas monicavignoliniluxury reddalsand defigners projektorilamput qtbservices labandas totalconceptdesign elannonnayttamo begmaterialiedili bardsdans samuelmanndds marksmenmfg demo17 chinafinewines coralia ivar-moe tournailesbains consew luisaprofumeriashop chimneycompanywestchester danapoly 268dental uspaerospace melonerp matcoservice emperorsoft cantare fninc greenpowerchemical westendsupply domobios unitysurfacingsystems martemoen hamptonssepticservices housatonicpaper sj-transport epsl-tunisie sungoldabrasives collinscreative barbarottomachinery soep thebestofalexandria straightlineconst itcimpianti hattrem-trafikkskole federalnetworks ppattorneys ceteau fbperformance coltgateway coolservice4u garaconfection bellformalwear abcconcretepumping mysantaria accurateindustrialmachining schmugerhardware thebestofjackson bellwetherstaffing sweetkarmadesserts tonerhuset mongilschool polycliniquelaouani buonidentro schoolbusmirrorsonline qlstransportation andereuropa cleanpressiondrycleaner bsyd planetlimony thebestoflasvegas csgmfoodequip potensial potiez invitiing tomscorvetteshop thebestbaltimorebusinesses honefoss thebestofoakland hydeparkdenim scmanndds sirreal totalpreferredsupply futureshockcorp myrtun
Keywords
Extractables and leachable, Container closure system, Analytical evaluation threshold, Safety concern threshold, Active pharmaceutical ingredient, Gas chromatography-mass spectroscopy
Introduction
The Due to several advantages’ primary CCS are widely used to store and transport most of the ophthalmic solutions is plastic [1]. The plastic has additional benefit that the dose can be administered in controlled manner with low variability in comparison to glass. The CCS should be evaluated systematically to identify the anticipated leachable which may affect the safety and efficacy of drug product [2-7]. The quality of material of construction of certain primary CCS for multi dose preservative free eye drops is extensively reviewed by Allison Campolo et al [8]. Certain official guidelines are provided to implement control strategy of packaging components [9,10]. Due to permeability nature of CCS and close contact of liquid drug products, leachable from CCS cannot be avoided. Extractables from CCS are studied by several research scholars [11,12]. Due to difference in toxicity the leachable has significant impact on patient safety. Certain ingredients of drug product may react with leachable which in turn converts in reaction products and enhance the risk to patient due to adverse effects [13-18]. Hence, control strategy of leachable from CCS in drug product is extremely important; however, development of suitable control procedure sometimes becomes tedious process due to adsorption phenomena of leachable on CCS, low concentration of drug substance, difference in permeability, drug saturation due to water loss, detection wavelength, techniques selected, masking by the drug product matrix and inherent product stability [19]. However, as a part of a risk assessment, it is necessary toidentify the leachable using sensitive method and to ensure that the drug product is suitable for their intended use. The review by Gagandeep et al provides an overview of certain analytical challenges encountered during E and L analysis [20]. As an additional protection, due to ease of handling and for marketing purpose, primary CCS are packedin secondary packaging components. The product label is fixed on immediate CCS. The labels mostly contain different inks, additives, solvents, plasticizers, photo initiators and activators. Due to long-term contact of drug product with immediate CCS, permeability and volatility nature of components from product label there is possibilityof migration of leachable in drug product [21-23]. Such anticipated leachable are evaluated during selection of immediate CCS through extractables study. While executing E and L study not only the components from immediate CCS, but their reaction/degradation products should be evaluated [24]. The leachable above AET need to be monitored till end of the shelf life. The development of control procedure is challenge due to low content of leachable however,the risk is high due to its direct exposure to human eye and difference in toxicity, carcinogenicity, and genotoxicity. As a result, the United States Pharmacopeia (USP) has included a chapter to provide guidance on control procedure of leachable.
Generic drug product of ophthalmic dosage form similar to “Xalatan” was selected for this invention. An in-house analytical procedure was developed for quantification of previously identified one of the leachable (Benzophenone). Apart from related substance, the stability study of the drug product was monitored typically for content of benzophenone. The sample chromatogram indicated that, the peak response due to one of the unknown components increased on time. The PDA spectra of the peak was compared with that of drug substance and identified impurities. The PDA spectra found different than drug substance and identified impurities hence, this peak was categorized as probable leachable. The source of this leachable was identified as ink component used to print product label. The leachable was isolated using preparative HPLC. Since, this leachable was assumed that it is volatile in nature hence GC-MS method was developed using several fractions collected from preparative HPLC. The molecular mass of the leachable was identified. The structure was elucidated and finally, the migrated compound was identified as “2- hydroxy-2-methyl-1- phenyl propane-1-one”. On further literature search it apprehended that, this compound is generally used as a photo initiator.
Materials and Methods
Leachable by HPLC (For Benzophenone)
HPLC grade prefiltered acetonitrile (ACN) and trifluoracetic acid (purity>99%) were purchased form Merck. The ultra-pure water obtained through MilliQ water system (Millipore, France) was used. Formic acid for preparative HPLC and deuterated chloroform for NMR studies were purchased from Merck.The standards (purity .99%) were purchased from authorized standard suppliers. HPLC column with dimensions 150 mm × 4.6 mm × 5 μm, Waters symmetry was used. The column temperature was selected as 30℃ and sampler temperature was set as 25℃. The detector wavelength was selected as 254 nm. Flow rate was maintained at 1 mL/minute. Injection size was selected as 100 μL.
Analysis of silver color
The major portion of colour of the product label is silver colour. Previously, another leachable (benzophenone) observed due to silver colour. Hence, the same lot of silver colour which was used to printthe product label was obtained from label printer. The identification solution of silver colour was prepared and injected in to HPLC as per method reported in section.
Preparative HPLC
Water’s preparative HPLC model 2767, 2998, CFO, 2525 was used. 0.5% formic acid in water was used as mobile phase-A. Prefiltered acetonitrile was used as mobile phase-B. Suitable gradient programme was developed. Inertsil ODS-3, 100 mm × 30 mm, 5 μm size preparative HPLC column was used to get adequate separationbetween adjacent peak and peak of interest. Flow rate was maintained at 25 mL/minute. The detector wavelength was selected as 254 nm. Injection volume was selected as 4500 μL.
GC-FID and GC-MS
Perkin Elmer GC model Clarus 500 was used. GC column DB-624, 30 m, 0.32 mm × 1.8 μm was used to get adequate separation between the adjacent peak and peak of interest. Helium gas was used as carrier gas.Flow rate was maintained at 1.3 mL/minute. Injection volume was selected as 0.5 μL. The quadrupole detector was used with EI+ as ion mode during determination of mass of peak of interest.
Structural elucidation
The FTIR manufactured by Perkin Elmer with spectrum mode using KBR technique was used to record FTIR spectrum. The GC-MS manufactured by Thermo with mass detector was used to record mass spectra. The NMR manufactured by Bruker, Model 400 ultra-shield was used to record chemical shifts of carbon atoms by dissolving isolated compound in CDCl3 and C13 NMR spectra was recorded.
Results and Discussion
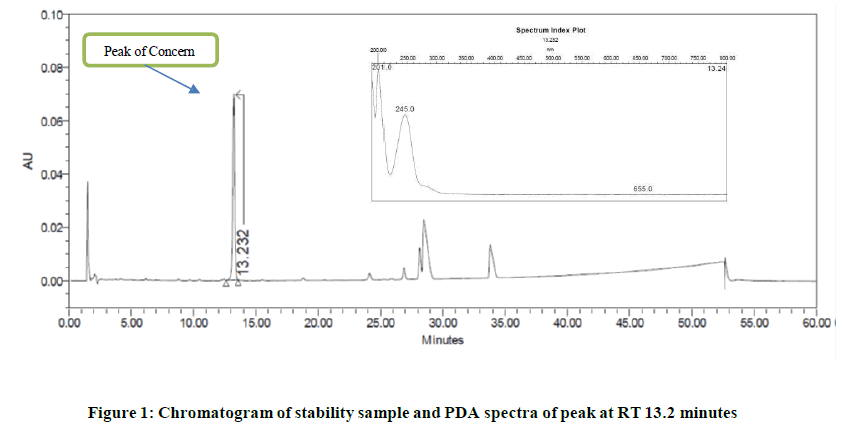
As a regulatory requirement, bioequivalent generic medicinal ophthalmic product having qualitative and quantitative composition similar to that of innovator “Xalatan” was manufactured. The drug product was stored as per ICH recommendations to evaluate the shelf life and storage statement. The method of analysis for leachable (specifically for benzophenone) was developed and included as a critical quality attribute during product stability study. During stability study another peak at retention time about 13.2 minutes found increasing on time in leachable method. The PDA spectrum of this peak found different than that of drug substance and identified impurities. The difference in PDA spectra, appearance of peak in leachable method, absence of this peak in placebo, drug substance, and spiked sample solution with identified impurities and the data of several batches suggests that the peak is not related to drug substance. Figure 1 indicates the chromatograms and PDA spectra of leachable of interest (Figure 1).
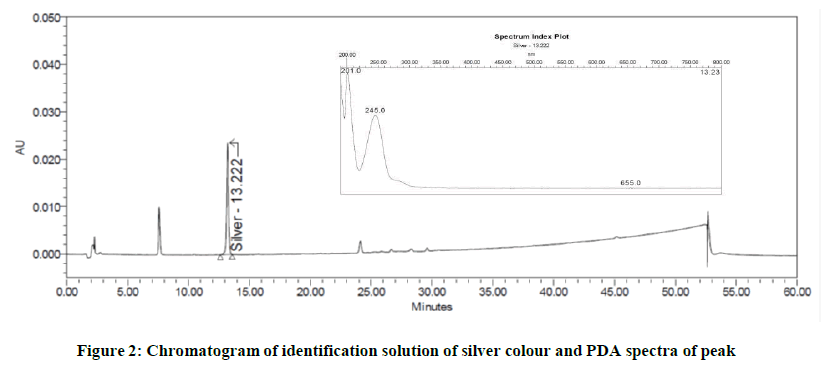
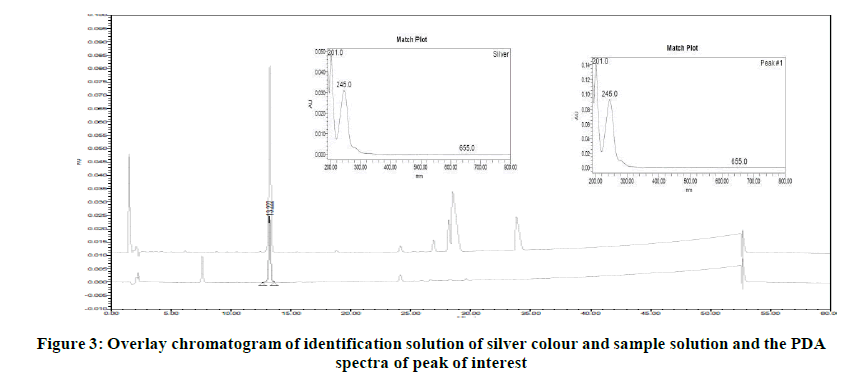
The chromatograms generated during development phase of leachable method was checked and it noticed that the label component is showing this peak at non-significant level. Since, this peak was very small the identification of source of this peak was not initiated during development phase. Based on this information, it suspected that the peak may be due to another leachable. As a confirmation, the identification solution of silver colour was injected into HPLC system in leachable method. The peak at RT about 13.2 minutes observed in this chromatogram. The PDA spectra of the peak in silver colour found similar to that obtained in sample solution. Figures 2 and 3 indicates the chromatograms of identification solution of silver colour and drug product. The PDA spectra of peak of interest is also reported in following (Figures 2 and 3).
Above study indicated that; the component of silver colour is responsible for this leachable. Hence, the compound elutes at RT about 13.2 minutes from silver colour was initiated to isolate using preparative HPLC. The method was developed to separate the closely eluting peak and the peak of interest. Multiple fractions were collected. Each fraction was injected in to HPLC coupled with PDA detector using the method reported in section 2.1 to confirm theretention time, peak purity and to check the similarity of spectral behavior for each fraction. The PDA spectra was of each fraction was compared with that obtained for the peak in the chromatogram of sample solution. Using mixture of several fractions, the compound was isolated. Figure 4 indicates chromatograms of isolated compound and the PDA spectra of the peak obtained due to isolated compound (Figure 4).
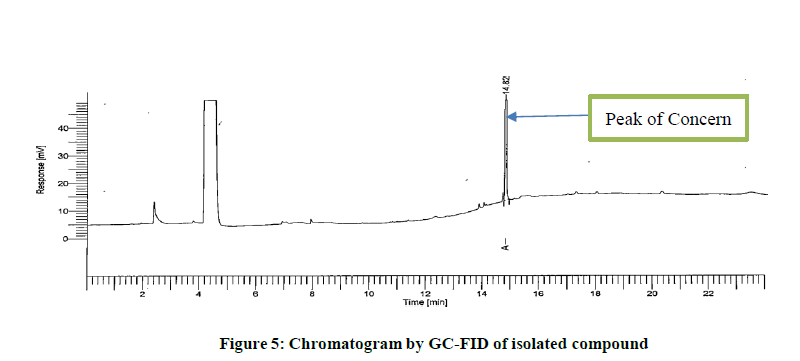
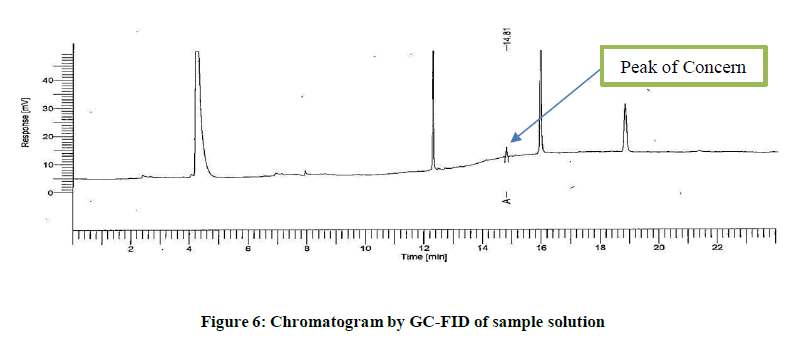
PDA spectra is not sufficient to conclude the leachable hence, separate analytical method was developed using GC-FID as reported in section 2.4. Initially, the isolated compound was analysed. Simultaneously the sample solution was also analysed to identify retention time. The retention time observed in chromatogram of sample solution coincides with that in chromatogram obtained due to isolated compound. This confirms the isolated compound is probably same as that obtained in chromatogram of sample solution. Figures 5 and 6 indicates the chromatogram of isolated compoundand the chromatogram of sample solution (Figures 5 and 6).
The FTIR spectrum of isolated compound was measured using KBr technique<USP 197K>. Based on the stretching, of
-OH, C=O, C=C and -C-H the compound identified as 2-hydorxy-2-methiyl-phenylpropane-1-one. The FTIR spectrum was further compared with that available in literature of Sigma-Aldrich [CAS No. 7473-98-5]. It noticed thatthe FTIR spectrum found concordant with the standard of Sigma-Aldrich. This confirms the peak elutes at retention time about
13.2 minutes is due to 2-hydorxy-2-methiyl-phenylpropane-1-one. To re-confirm the identification the chemical shift (δ) using H1 NMR was checked, and the assignment is reported in Figure 7.
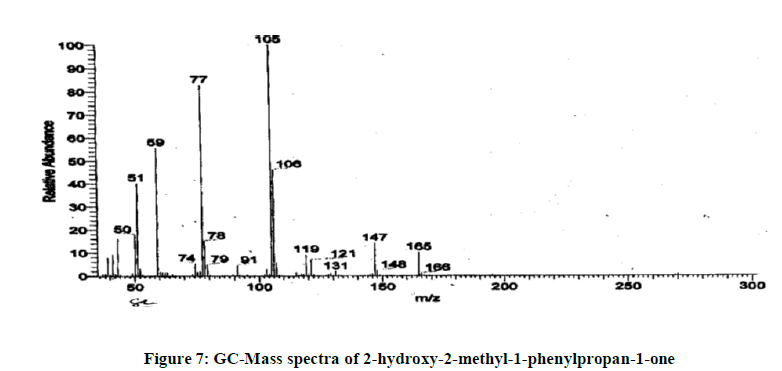
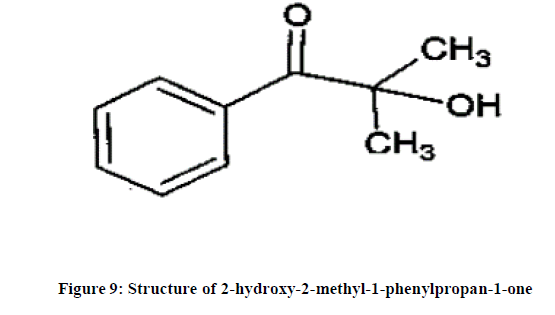
The spectrum using C13 NMR was also recorded and based on chemical shift (δ), the position of carbon atoms was assignedas reported in Figure 8. The depth data shows that carbon atoms are at positions 1,7 and 9 which are quaternary carbon atoms as reported in Figure 9. GC-MS data confirmed the molecular weight of compound is 164 which co- relates with theoretically calculated molecular mass. Overall, this leachable is confirmed as 2-hydorxy-2-methiyl- phenylpropane-1- one. The GC-MS spectra and the structure elucidated using all these techniques are reported in Figures 7-9.
Additional literature was searched and noticed that; this compound is commonly used as photo initiator for various applications. The compound is non carcinogenic and non-genotoxic is nature. This compound is commercially available as Irgacure R 1173 as a brand name. The MSDS mentions that the compound is non-irritant to human eye and skin. The assessment of maximum allowable concentration of compound was calculated as per PQRI recommendations. Thedaily exposure of ophthalmic drug product is 2 drops per day. For ophthalmic solution, the weight of a drop is about 33 μL. Considering both the eyes are affected the daily dose of ophthalmic drug product is 0.066 mg/day. The limit for this leachable was arrived at 2.27 ppm. Finally, to ensure the patient safety, the analytical method is validated usingthis compound. The method was implemented as a critical quality attribute to release and evaluation of shelf life of the product. In addition to this all the commercial batches available in market were analysed. The data of all batches showed that, the content below qualification threshold which ascertain the patient safety. The structure of the compound elucidated using QSAR VEGA software for isolated compound.
Conclusion
Development of quality control procedure to estimate potential leachable is challenging especially for ophthalmic dosage forms due to low content of drug substance. The product specific assessment of leachable is very important due to difference in toxicity, its reactivity with product matrix and may generate complex and more hazardous degradation product. Leachable from secondary packaging components are typically due to its volatility in nature. Hence, assessment of label components is necessary to understand the anticipated leachable in liquid dosage forms. An expectation by regulators for selection of control procedure of leachable is notified to pharmaceutical manufacturers on timely manner to ensure the patient safety. There are large number of experimental designs publicly available which can adapt to select immediate container closure system. However, this study design is very effective and can be implemented as a tool during prototype development, development of control procedure and to accumulate enough knowledge about anticipated leachable from secondary CCS. This holistic approach can also be used to develop control procedure and specification during manufacturing of secondary CCS. This study design further provides information about systematic approach to be followed during selection of semi migrant/non migrant labels, quality of ink and to design the product label. Recently the expectations from regulators have increased to include related substances published in scientific journals hence, this article will help applicant to design product specification. Overall, this study design helps to ascertain patient safety during product development phase itself and to avoid anticipated commercial product recalls.
References
- Santvliet LC, Ludwig A. SurveyofOphthalmology. 2004; 49(2): 197-213. [Cross Ref]
- Vardakou I, Karampela S, Papoutsis I, et al. J.Anal.Chem. 2014; 69(1): 1096–1101.
- Luo Y, Rank M, Fujimori K, et al. Biopharma Asia. 2018; 30-36.
- Hammond M, Marghitoiu L, Lee H, et al. Biotechnol.Prog. 2014; 30(2): 332-337.
- Liu D, Nashed SY, Bondarenko PV, et al. PDAJPharmSciTech. 2012; 66(1): 12-19.
[Cross Ref][Google Scholar ] [Pub Med]
- Hammond M, Nunn H, Rogers G, et al. PDAPharmSciTech. 2013; 67(2): 123-134.
[Cross Ref][Google Scholar ] [Pub Med]
- Jenke D. Tr.AC-TrendsinAnal.Chem. 2018; 101(1): 56-65.
- Campolo A, Crary M, Shannon P. Biomed.j.sci.technol. res. 2022; 45(1): 36035- 36044.
- Guidance for Industry: Container closure systems for packaging human drugs and biologics. 1999.
- Santvliet LC, Ludwig A. SurveyofOphthalmology. 2004; 49(2): 197-213.
- Zhiang X, Lei S, Zou M, et al. J.Pharm.Biomed. 2022; 220(1): 115015.
- harm.Biomed. 2023; 236(1): 115640.[ Cross Ref]
[Google Scholar] [Pub Med]
- Burman L, Albertsoon AC, Hoglund A. J.Chromatogr.A. 2005; 1080(2): 107-116.
- Zokande S, Inamdar K, Kale A. WorldJPharmPharmSci. 2023; 12(4)1600-1607.
- Zokande S, Inamdar K, Mane V. WorldJPharmPharmSci. 2023; 12(10): 959-972.
- Rodriguez LC, Muros ML, Samblas CR, et al. Int.J.Pharm. 2020; 583(1): 119332.
- Broschard TH, Glowienke S, Bruen US, et al. Regul.Toxicol.Pharm. 2016; 81(1): 201–211.
- Jenke D, Carlson T. PDA J. Pharm. Sci. Technol. 2014; 68 (5): 407–455.
[Cross Ref][Google Scholar] [Pub Med]
- Roy J. AAPSPharma SciTech. 2002; (2): 1-8.
- Singh G, Lu D, Liu C, et al. TrendsAnalytChem. 2021; 141(1): 116286.
- Paskiet D, Jenke D, Ball D, et al. PDAJPharmSci Technol. 2013; (67): 430-447.
[Cross Ref][Google Scholar] [Pub Med]
- Fu C, Jiang F, Gao K, et al. J. Pharm. Biomed. 2023; 235(1): 115591.
- Gollapalli R, Singh G, Blinder A, et al. J.Pharm.Sci. 2019; 108(10): 3187-3193.
[Cross Ref][Google Scholar] [Pub Med]
- International Conference on Harmonisation. Q1A(R2): Stability testing of new drug substances and products. 2003.