Original Articles: 2024 Vol: 16 Issue: 3
Green Synthesis of Pyrazolines as an Anti-Inflammatory, Antimicrobial and Antioxidant Agents
Kiran Pawar*, Sachin Bhosale, Avinash Dhake, Nirmala Shinde
Department of Chemistry, SMBT College of Pharmacy, Maharashtra, India
- Corresponding Author:
- Kiran Pawar
Department of Chemistry,
SMBT College of Pharmacy,
Maharashtra,
India
Received: 02-Jul-2023, Manuscript No. JOCPR-23-104545; Editor assigned: 05-Jul-2023, PreQC No. JOCPR-23-104545 (PQ); Reviewed: 19-Jul-2023, QC No. JOCPR-23-104545; Revised: 27-Dec-2023, Manuscript No. JOCPR-23-104545 (R); Published: 03-Jan-2024, DOI:10.37532/0975-7384.2024.16(01).073.
Citation: Pawar K, et al. 2024. Green Synthesis of Pyrazolines as an Anti-Inflammatory, Antimicrobial and Antioxidant Agents. 16:073.
Copyright: © 2024 Pawar K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Keywords
Pyrazoline, Antimicrobial, Antioxidant, Anti-inflammatory, DPPH
Introduction
In recent years, green chemistry has been focus of considerable attention and is becoming an increasingly popular technology [1]. The aim of green chemistry is to reduce chemical related impacts on human health and virtually eliminate contamination of the environment through dedicated sustainable prevention program. For organic reactions there is an urgent need for reducing toxic, volatile solvents in use. Many green synthetic methods have been studied by different researchers for the synthesis of a variety of organic molecules [2]. Microwave irradiation is convenient synthetic method and continues to affect synthetic chemistry significantly by enabling rapid, reproducible, and scalable chemistry development [3]. This technique has been applied to a variety of reactions resulting in reduction of reaction time, higher yield, greater selectivity, cleaner reaction products, and easier manipulations [4,5]. It also provides an opportunity to work with open vessels, thus avoiding the risk of high pressure and hazards of inflammable solvents [6]. We herein report microwave assisted synthesis access to a series of chalcones and their conversion to heterocycles (2a-i and 3a-i) from substituted benzaldehyde and substituted acetophenone demonstrate its superiority over classical heating methods [7-9].
It is worth mentioning that chalcones are the precursor of flavonoids, isoflavonoids, and have displayed an impressive array of biological activities [10,11]. Particularly, chalcones have been reported as anti-inflammatory, ant proteolytic, antioxidant, antibacterial, anticancer and antiproliferative. Compared to conventional approaches, the grinding process is one that is being employed more and more in organic synthesis. These reactions are interesting from an economic standpoint, most frequently provide significant benefits in terms of yield, selectivity, and ease of reaction procedure with high atom efficiency [12].
MATERIALS AND METHODS
Materials
The starting materials and solvents used for each reaction are of synthetic grade procured from Sigma Aldrich. All the chemicals and synthesized products were characterized for their purity by physical constant and Thin Layer Chromatography (TLC). All the reactions were monitored by using a pre-coated TLC plates (silica gel 60-120). Solvent system chloroform: methanol (8:2) for step-1 compounds and chloroform: methanol (9:1) for step-2 compounds. The obtained TLC plates were observed under a long UV lamp in a UV chamber and also in iodine chamber for detection of spots. All reactions were carried under microwave irradiation and grinding technique by using microwave make, Labline scientific instruments. The synthesized compounds were characterized by spectroscopic method FTIR, mass and 1H, 13C NMR for elucidation and confirmation of their structures [13].
Methods
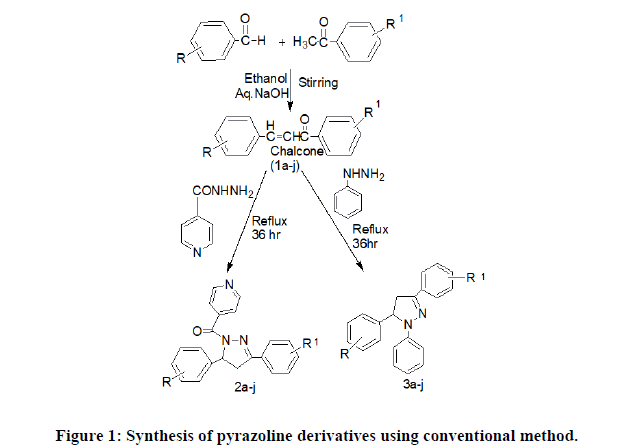
Conventional heating method (1a-i): Step 1: Placed a solution of 2.2 gm of sodium hydroxide in 20 ml of water and 12.5 ml of ethanol in conical flask with magnetic stirrer. The flask was kept in crushed ice. Then (0.43 Mol) 4.4 ml of acetophenone was added in the above mixture by continuously stirring with magnetic stirrer. Then added a substituted benzaldehyde (0.43 mol) 4.6 ml dropwise and maintain the temperature of the blend upto 25°C. The reaction mixture was kept in a refrigerator overnight [14,15]. Further the reaction mixture was diluted with 50 ml of cold water and acidified with 10% aq. HCl to precipitate the chalcone. Then the obtained product was filtered and washed with cold water until the washing were neutral to litmus and further with 10ml of cold rectified spirit. Then the product was recrystallised using chloroform [16].
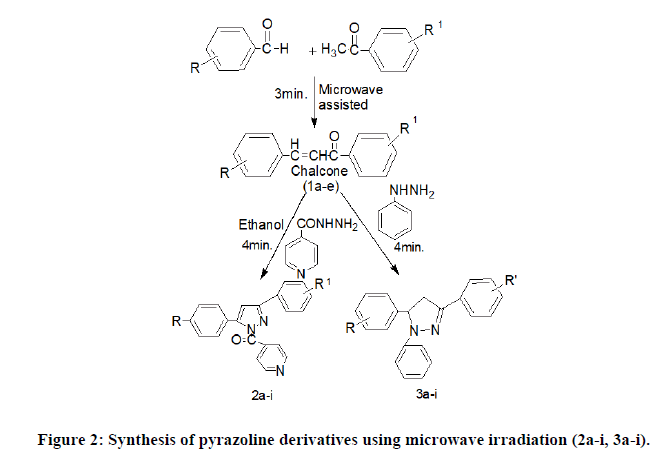
Microwave irradiation method (1a-i): Step 1: Substituted acetophenone (5 mmol) and substituted benzaldehyde (5 mmol) 1N NaOH 1N (0.01 mol) were dissolved in ethanol (7.5 mL) in the round bottom flask. The mixture was irradiated by using microwave for 3 min at 180 W and monitored by using TLC and neutralized by adding 3N HCl (5 ml). The product was filtered and washed with cold water [17].
Step 2: General procedure for the synthesis of pyrazolines derivatives (2a-i, 3a-i): Substituted chalcone 1a-i (10 mmol) and INH (10 mmol, 1.37 g) for 2a-i /phenyl hydrazine hydrate (10 mmol, 1.5 g) 3a-i were dissolved in 10 ml glacial acetic acid and refluxed for 36 h. The progress of reaction was monitored by TLC. The mixture was poured into ice cold water and neutralized with sodium bicarbonate. The sticky nature was removed by treatment with brine if necessary (Figure 1 and Table 1) [18].
| Compounds | R (substituted benzaldehyde) | R’(substituted acetophenone) | ||
|---|---|---|---|---|
| 1a | 2a | 3a | m-nitro | p-methyl |
| 1b | 2b | 3b | 3,4,5-trimethoxy | |
| 1c | 2c | 3c | m-hydroxy | |
| 1d | 2d | 3d | p-fluoro | |
| 1e | 2e | 3e | 3,4,5-trimethoxy | p-chloro |
| 1f | 2f | 3f | m-nitro | |
| 1g | 2g | 3g | m-hydroxy | |
| 1h | 2h | 3h | p-fluoro | |
| 1i | 2i | 3i | p-nitro | |
Table 1: Pyrazoline derivatives using conventional method.
Step 3: General procedure for the synthesis of pyrazolines derivatives (2a-i, 3a-i): Comp. 2a-i were synthesized by microwave irradiation for 3-5 min. by adding compound (1a-i) (2.5 mmol) with isonicotinic acid hydrazide (2.5 mmol) dissolve in glacial acetic acid respectively.
Similarly, 3a-i were synthesized by adding comp. (1a-i) with phenyl hydrazine (2.5 mmol) in an open pyrex vessel and irradiated in a microwave oven for the appropriate time (3-5 min) until the completion of reaction (monitored by TLC). After completion, the reaction mixture was cooled at room temperature and was poured into cold water (50 ml). The solid as obtained was filtered, dried, and recrystallised from methanol (Figures 2 and 3).
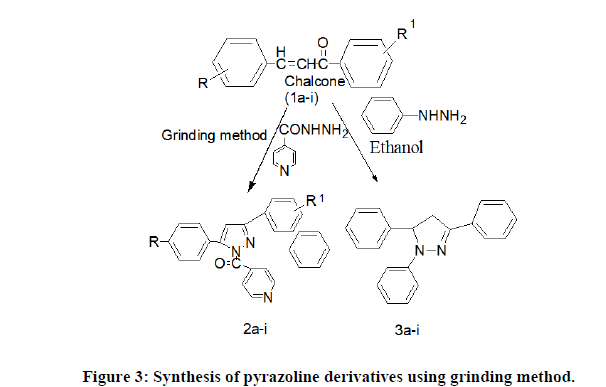
General method for synthesis of pyrazoline derivatives using grinding method (2a-i, 3a-i)
Step 1: Performed same as given in procedure of microwave synthesis.
Step 2: Synthesis of comp. 2a-i were synthesis by adding comp. 1a-i (0.01 mol) with isoniconic acid hydrazide hydrate (0.02 mol) dissolve in glacial acetic acid was added to this reaction mixture and grinding continued for several minutes, completion of reaction monitored by TLC, the light yellow-greenish coloured solid was separated out.
Similarly comp. 3a-i were synthesized by adding comp 1a-i with phenyl hydrazine hydrate (0.02 mol) 3a-i synthesis was thoroughly ground with a pestle in an open mortar at room temperature for 2-3 minutes. Acetic acid (0.001 mmol) was added to this reaction mixture and grinding continued for several minutes, completion of reaction monitored by TLC, the light yellow-greenish colored solid was separated out. The obtained solid was diluted with cold water and isolated by simple Buchner filtration and recrystallization from ethanol to give pyrazoline derivatives.
(5-(4-Nitro-phenyl)-3-p-tolyl-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2a): Dark brown powder, mol. formula-C23H19N3O3, mol. wt–382.50; IR cm-1 C-H stretch-3338.03, Sp3 C-H stretch-2920.23, C-N stretch-1243.55, C-NO2 stretch-1550 1HNMR 7.44 (s,1H, J=8.6 Hz), 7.95 (s, 1H, J=8.6 Hz), 7.51 (1H, J=7.6 Hz), 7.44 (1H, J=7.7 Hz), 7.95 (1H, J=6.7 Hz), 7.38 (1H, J=8.6 Hz), 8.14 (1H, J=7.4 Hz), 8.14 (1H, J=7.5 Hz,), 7.38 (1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 1.9 (d, CH2 1H, J=7.9/5.8 Hz), 2.35 (s, 1H, J=16.1/8.4 Hz), for 13C NMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+: Calcd. For C23H19N3O3: 382.50; found: 383.25.
2-methyl-1-(3-p-tolyl-5-(2,3,4-trimethoxy-phenyl)-4,5-dihydro-pyrazol-1-yl)-penta-2,4-dien-1-one (2b): Darkbrown powder, mol. formula-C26H26N2O, mol. wt–382.50; IR cm-1 Sp2 C-H stretch-2967, Sp3 C=O stretch-1688.5, C-N stretch-1326, N-H stretch-3336.0, C=C stretch-1593.20 1HNMR7.4 (d, 1H, J=8.6 Hz), 7.3 (t, 1H, J=8.6 Hz), 7.2 (t,1H, J=7.6 Hz), 7.2 (d, 1H, J=7.7 Hz), 7.2 (d, 1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.7 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5Hz,), 4.8 (dd, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS(ESI, m/z) (M+H)+ : Calcd. For C26H26N2O: 382.50; found: 381.5.
(5-(3-Fluoro-phenyl)-3-p-tolyl-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2c): Light brown powder; mol. formula-C23H19FN2O, mol. wt–358.15 ;IR cm-1 Sp2 C-H stretch-2875.85, Sp3 C-H stretch-2878.85, C-N stretch-1227, C=C stretch-1548, C-OH stretch-3324.43 1HNMR7.4 (d, 1H, J=8.6 Hz), 7.44 (1H, J=8.6 Hz), 7.51 (1H, J=7.6 Hz), 7.19 (d, 1H, J=7.7 Hz), 6.89(1H, J=6.7 Hz), 6.83 (1H, J=8.6 Hz), 6.79 (1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 2.35 (s, 3H), 1.9 (1H, J=7.9/5.8 Hz for 13CNMR 128.6, 131.9, 128.6, 127.3, 133.5, 127.3, 168, 168, 45.4, 20.9, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C23H19FN2O: 358.15; found: 357.
(5-(4-Hydroxy-phenyl)-3-p-tolyl-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2d): Dark brown powder, mol. formula-C23H20N2O2, mol. wt–356.15 ;IR cm-1 C-H stretch-2970.82, N-H stretch-338.15, C-N stretch-1245.25, C=C stretch-1519.78 1HNMR 7.95 (1H, J=8.6 Hz), 7.51 (1H, J=8.6 Hz), 7.44 (1H, J=7.6 Hz), 7.95 (1H, J=7.7 Hz), 6.8 (1H, J=6.7 Hz), 7.1 (1H, J=8.6 Hz), 6.68 (d, 1H, J=7.4 Hz), 7.5 (1H, J=7.5 Hz,), 4.9 (1H, J=10.3/8.5 Hz), 2.35 (s, 3H), for 13CNMR 155.6, 155.3, 168, 128.6, 115.5, 135.0, 128.5, 128.6, 131.9, 133.5, 128.2, 140.1, 42.7,20.9 ; HRMS: MS (ESI, m/z) (M+H)+: Calcd. For C23H20N2O2: 356.15; Found: 355.16.
(3-(4-Chloro-phenyl)-5-(2,3,4-trimethoxy-phenyl)-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2e): Brick red powder, mol. formula-C25H23ClN2O4, mol. wt–450.91;IR cm-1 Sp2 C-H stretch-2958.80, Sp3 C-H stretch-2835.36, C-N stretch-1244.09, C-O stretch-1282.66, C=C stretch-1581.63, C-Cl stretch-779.24 1HNMR 7.44 (d, 1H, J=8.6 Hz), 7.5 (t, 1H, J=8.6 Hz), 7.95 (t, 1H, J=7.6 Hz), 7.6 (d, 1H, J=7.7 Hz), 7.3 (d, 1H, J=6.7 Hz), 7.6 (d, 1H, J=8.6 Hz), 6.17 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.9 (d,1H, J=10.3/8.5 Hz), 1.9 (s, 3H), 3.75 ( 1H, J=7.9/5.8 Hz), for 13CNMR 168, 155.6, 129.3, 127.3, 127.3, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 128.4, 56.6, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C25H23ClN2O4: 450.91; found: 449.5.
(-(4-Chloro-phenyl)-5-(4-nitro-phenyl)-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2f): Yellowish brown powder, mol. formula-C22H16ClN3O3, mol. Wt–405.09; IR cm-1 Sp2 C-H stretch-3050.39, Sp3 C-H stretch-2930.94, Ter C-N stretch-1016.56, C-F stretch-1128.43, C=C stretch-1590; 1HNMR 8.14 (1H, J=8.6 Hz), 7.38 (1H, J=8.6 Hz), 7.3(1H, J=7.6 Hz), 7.95(1H, J=7.7 Hz), 7.44 (1H, J=6.7 Hz), 6.7 (d, 1H, J=8.6 Hz), 8.14 (1H, J=7.4 Hz), 4.8 (1H, J=10.3/8.5Hz), 3.9 (s, 3H), for 13CNMR 155.6, 153.9, 168.2, 134.9, 130.4, 129.0, 127.3, 133.5, 125.4, 146.4, 62.2, 55.4, 42.7;HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C22H16ClN3O3: 405.09; found: 403.5.
(3-(4-Chloro-phenyl)-5-(4-hydroxy-phenyl)-4,5-dihydro-pyrazol-1yl)-phenyl-methanone (2g): Brown powder, mol. formula-C22H17 ClN2O2, mol. wt–376.84 ;IR cm-1 OH stretch- 3335.06, Ar-CH stretch-3075, Ali CH stretch-2967, C=O stretch-1650, C=N stretch-1585, C-O stretch-1127, C-Cl stretch-830.47;1HNMR 7.6 (1H, J=8.6 Hz), 7.3 (1H, J=8.6 Hz), 7.44 (1H, J=7.6 Hz), 7.6 (1H, J=7.7 Hz), 7.95 (1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.68 (1H, J=7.4 Hz), 5.0 (OH, J=7.5 Hz, for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C22H17ClN2O2: 376.84; found: 367.5.
(3-(4-Chloro-phenyl)-5-(3-fluoro-phenyl)-4,5-dihydro-pyrazol-1-yl)-phenyl-methanone (2h): Reddish brown powder, mol. formula-C22H16ClFN2O, mol. wt–376.83; IR cm-1 OH stretch-3365, Ar-CH stretch-3065, Ali CH stretch-2935, C=O stretch-1735, C=N stretch-1611, C-O stretch-1125; 1HNMR 7.4 (1H, J=8.6 Hz), 7.79 (1H, J=8.6 Hz), 7.5 (1H, J=7.6 Hz), 7.44 (1H, J=7.7 Hz), 7.95 (1H, J=6.7 Hz), 6.8 (1H, J=8.6 Hz), 6.7 (1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.8 (dd, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C22H16ClFN2O: 376.83; Found: 268.14.
(3-(4-Chloro-phenyl)-5-(3-nitro-phenyl)-4, 5-dihydro-pyrazol-1-yl)-phenyl-methanone (2i): Dark brown powder; mol. formula-C22H16 ClN3O3, mol. wt–405.83 ;OH stretch-3326, CH stretch-3059, Ali CH stretch-2948, C=O stretch-1744, Ar-CH stretch-3059, C=N stretch-1625, C-O stretch-1122; 1HNMR 7.4 (1H, J=8.6 Hz), 7.3 (1H, J=8.6 Hz), 7.29 (1H, J=7.6 Hz), 7.2 (1H, J=7.7 Hz), 7.2 (1H, J=6.7 Hz), 6.9 (1H, J=8.6 Hz), 6.7 (1H, J=7.4 Hz), 6.7 (1H, J=7.5 Hz,), 4.8 (1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (d, 1H, J=7.9/5.8 Hz), 3.15 (1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 151.2, 144.5, 135.6, 135.5, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C22H16ClN3O3: 405.83; found: 268.144.
5-(3-nitro-phenyl)-1-phenyl-3-p-tolyl-4,5-dihydro-1H-pyrazole (3a): Brown powder, mol. formula-C22H19 N3O2, mol. wt–357.41 ;IR cm-1 Sp2 C-H stretch-2967, C=O stretch-1688.5, C-N stretch-1326.9, C-NO2 stretch-1350, C=C stretch-1517.20, 1613.9, N-H stretch-3336.0 ;1HNMR 7.407 (1H, J=8.6 Hz), 8.01 (1H, J=8.6 Hz), 8.05 (1H, J=7.6 Hz), 7.51 (1H, J=7.7 Hz), 7.2 (1H, J=6.7 Hz), 6.5 (1H, J=8.6 Hz), 4.8 (d, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), for 13CNMR 159.1, 153.9, 152.6, 130.5, 140.5, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 40.8; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C22H19N3O2: 357.41; found: 268.1441.
1-phenyl-3-p-tolyl-5-(3,4,5-trimethoxy-phenyl)-4,5-dihydro-1H-pyrazole (3b): Reddish brown powder; mol.formula-C25H26N2O3, mol. wt–405.49; OH stretch-3343, ArCH-2934, C=N stretch-1645, C-NO2-1572, CO stretch-1120, N-H stretch-1329, C=O stretch-1739 ;1HNMR 9.2(1H, J=8.6 Hz), 8.38 (1H, J=8.6 Hz), 8.10 (1H, J=7.6 Hz), 7.4(1H, J=7.7 Hz), 6.8 (1H, J=6.7 Hz), 6.72 (d, 1H, J=8.6 Hz), 5.82 (1H, J=7.4 Hz), 4.28 (1H, J=7.5 Hz,), 4.8 (1H, J=10.3/8.5Hz), 3.32 (s, 3H), 3.28 (s, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 165.7, 151.9, 133.5, 149.5,128.3, 126.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. ForC25H26FN2O3: 405.49; found: 404.5.
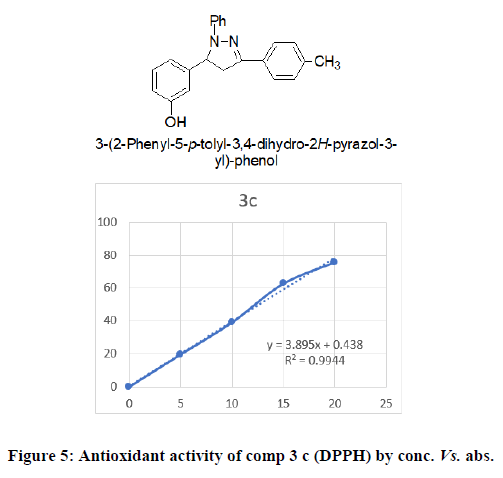
3-(2-phenyl-5-p-tolyl-3,4-dihydro-2H-pyrazol-3yl)-phenol (3c): Brick red powder, mol. formula-C22H20N2O, mol. wt–328.41;IR cm-1 Sp2 C-H stretch- 3031.80, Sp3 C-H stretch-2950.50, Ter C-N stretch-1285.50, C-NO2 stretch-1350.17, C=C stretch-1596.99, C-Cl stretch-695.30 ;1HNMR 7.4 (d, 1H, J=8.6 Hz), 7.3 (t, 1H, J=8.6 Hz), 7.2 (t, 1H, J=7.6 Hz), 7.2 (d, 1H, J=7.7 Hz), 7.2 (d, 1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.7 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.8 (dd, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+: Calcd. For C23H19FN2O: 267.3258; found: 268.1441
5-(4-fluoro-phenyl)-1-phenyl-3-p-tolyl-4,5-dihydro-1H-pyrazole (3d): Dark brown powder, mol. formula- C22H19FN2, mol. w–330.40 ;IR cm-1 Sp2 C-H stretch-3037.89, Sp3 C-H stretch-2920.23, Ter C-N stretch-1219, C-NO2 stretch-1315.17, C=C stretch-1598.99, C-Cl stretch-696.30; 1HNMR7.4 (d, 1H, J=8.6 Hz), 7.3 (t, 1H, J=8.6 Hz), 7.2 (t, 1H, J=7.6 Hz), 7.2 (d, 1H, J=7.7 Hz), 7.2 (d, 1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.7 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.8 (dd, 1H, J=10..3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C23H19FN2O: 267.3258; found: 268.1441
3-(4-Chloro-phenyl)-1-phenyl-5-(3.4.5-trimethoxy-phenyl)-4,5-dihydro-1H-pyrazole (3e): Light brown powder, mol. formula-C24H23ClN2O3, mol. wt–422.90 ;IR cm-1 Sp2 C-H stretch-2958.80, Sp3 C-H stretch-2835.36, C-N stretch-1244.09, C-O stretch-1282.66, C=C stretch-1581.63, C-Cl stretch-779.24; 1HNMR7.4 (d, 1H, J=8.6 Hz), 7.3 (t, 1H, J=8.6 Hz), 7.2 (t, 1H, J=7.6 Hz), 7.2 (d, 1H, J=7.7 Hz), 7.2 (d, 1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.7 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.8 (dd, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C23H19FN2O: 267.3258; found: 268.1441.
3-(4-Chloro-phenyl)-5-(3-nitro- phenyl)-1-phenyl-4,5-dihydro-1H-pyrazole (3f): Dark brown powder, mol. formula- C21H16 ClN3O2, mol. wt–377.82; IR cm-1 Sp2 C-H stretch-3051.39, Sp3 C-H stretch-2927.94, Ter C-N stretch-1014.56, C-F stretch-1126.43, C=C stretch-1598. 1HNMR7.4 (d, 1H, J=8.6 Hz), 7.3 (t, 1H, J=8.6 Hz), 7.2 (t, 1H, J=7.6 Hz), 7.2 (d, 1H, J=7.7 Hz), 7.2 (d, 1H, J=6.7 Hz), 6.9 (d, 1H, J=8.6 Hz), 6.7 (d, 1H, J=7.4 Hz), 6.7 (d, 1H, J=7.5 Hz,), 4.8 (dd, 1H, J=10.3/8.5 Hz), 3.9 (s, 3H), 3.75 (dd, 1H, J=7.9/5.8 Hz), 3.15 (dd, 1H, J=16.1/8.4 Hz), for 13CNMR 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C23H19FN2O: 267.3258; found: 268.1441
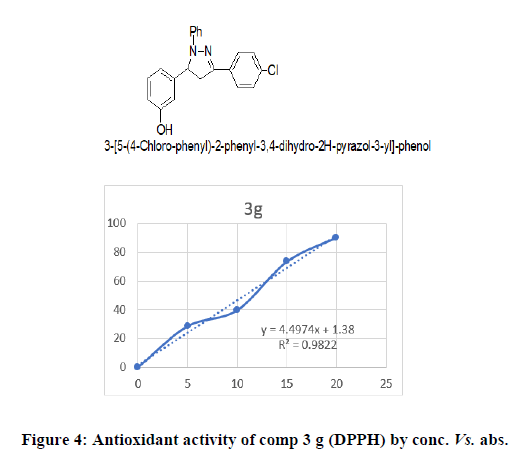
3-(5-(4-Chloro-phenyl)-2-phenyl-3,4-dihydro-2H-pyrazol-3-yl)-phenol (3g): Yellowish brown powder, mol. formula-C21H17ClN2O, mol. wt–348.83 ;IR cm-1 OH stretch-3333.06, Ar-CH stretch-3060, Ali CH stretch-2967, C=O stretch-1674, C=N stretch-1588, C-O stretch-1125, C-Cl stretch-825.47, 1HNMR 9.84 (1H, J=8.6 Hz), 7.85 (1H, J=8.6 Hz), 7.79 (1H, J=7.6 Hz), 7.63 (1H, J=7.7 Hz), 7.5 (1H, J=6.7 Hz), 7.31 (d, 1H, J=8.6 Hz), 6.87 (1H, J=7.4 Hz), 6.79 (1H, J=7.5 Hz,), 5.18 (1H, J=10.3/8.5 Hz), 3.9 (s, 3H), for 13CNMR 167.2, 151.6 159.1, 153.9, 146.2, 134.9, 130.0, 129.4, 127.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 62.2, 55.4, 42.7,38.3 ; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C21H17ClN2O: 348.83; found: 268.1441.
3-(4-Chloro-phenyl)-5-(4-fluro-phenyl)-1-phenyl-4,5-dihydro-1H-pyrazole (3h): Brown powder, Mol. Formula- C21H16 ClFN2, Mol.Wt–350.85 ;IR cm-1 OH stretch-3369, Ar-CH stretch-3066, Ali CH stretch-2938, C=O stretch-1737, C=N stretch-1601, C-O stretch-1123, 1HNMR 8.96 (1H, J=8.6 Hz), 7.84 (1H, J=8.6 Hz), 7.82 (1H, J=7.6 Hz), 7.71 (1H, J=7.7 Hz), 7.043 (1H, J=6.7 Hz), 6.84 (1H, J=8.6 Hz), 6.7 (1H, J=7.4 Hz), 6.711 (1H, J=7.5 Hz,), 5.65 (1H, J=10.3/8.5 Hz), 3.85 (s, 3H), 3.75 (1H, J=7.9/5.8 Hz), 3.15 (s, 1H, J=16.1/8.4 H-+z), for 13CNMR 168, 162.1, 151.1, 137.6, 134.8, 133.4, 131.6, 127.6, 116.4, 115.7, 114.1, 114.1, 113.9, 67.2, 55.4, 42.7; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C21H16FN2O: 350.85; found: 349.5.
3-(4-Chloro-phenyl)-5-(4-nitro-phenyl)-1-phenyl-4,5-dihydro-1H-pyrazole (3i): Reddish brown powder, mol. formula-C21H16 ClN3O2, mol. wt–377.8; OH stretch-3326, CH stretch-3059, Ali CH stretch-2948, C=O stretch-1744, Ar-CH stretch-3059, C=N stretch-1625 , C-O stretch-1122; 1HNMR 10.16 (1H, J=8.6 Hz), 8.98 (1H, J=8.6 Hz), 7.93 (1H, J=7.6 Hz), 7.65 (1H, J=7.7 Hz), 7.63 (1H, J=6.7 Hz), 6.8 (1H, J=8.6 Hz), 6.73 (1H, J=7.4 Hz), 5.65 (1H, J=7.5 Hz,), 4.8 (d, 1H, J=10.3/8.5 Hz), 3.4 (s, 3H), 3.75 (1H, J=7.9/5.8 Hz), 3.15 (1H, J=16.1/8.4 Hz), for 13CNMR 170.9, 167.6, 149.2, 148.9, 143.5, 137.2, 135.6, 132.9,112.5, 115.7, 114.1, 114.1, 112.9, 62.6, 57.9, 41.2; HRMS: MS (ESI, m/z) (M+H)+ : Calcd. For C21H16ClN3O2: 377.8; found: 376.5.
Biological activity
Antioxidant activity: DPPH radical scavenging method DPPH is a stable free radical widely used to assess the radical scavenging activity of antioxidant components. DPPH free radical has a stable violet colour in methanol and becomes colourless or yellow colour when paired with antioxidant or reducing agents. These radicals can accept the odd electron or hydrogen from the antioxidant and converted to a stable diamagnetic molecule (yellow). To identify the antioxidant activity, the solution of synthesised compounds has been prepared by dissolving in methanol (100 μg/ml). Simultaneously, a solution of DPPH was also prepared in another container had been shown maximum absorbance at 517 nm due to the presence of stable 1,1-diphenyl 2-picryl hydrazyl stable free radical (violet colour). Each test compound (4 ml) was added to 4 ml of DPPH solution and kept aside for 30 min at room temperature. Absorbance was measured at 517 nm using a Shimadzu ultraviolet spectrometer. Blank and standard absorbance were also calibrated. Antioxidant activity of ascorbic acid derivatives was determined through directly substituting the absorbance in the mentioned formula.
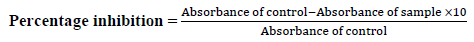
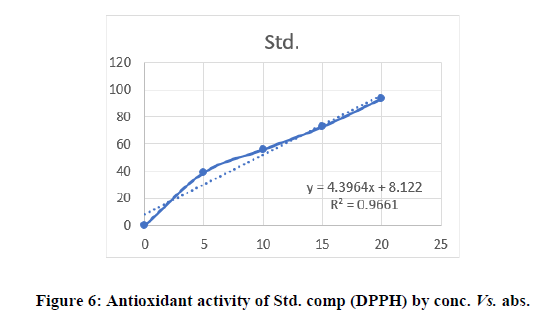
DPPH method: DPPH is a perfect method to evaluate the antioxidant activity of newly synthesised compounds due to the short time required for the analysis and high reliability. The result reveals that decreases the absorbance due to the antioxidant molecule may supply the electron to the DPPH free radical. Most of the compounds were shown excellent antioxidant activity. The anti-oxidant activity of synthesised compounds was shown in Table 2 (Figures 4-6).
| Sr.no. | Comp. code |
% Inhibition | ||||
|---|---|---|---|---|---|---|
| 5 µg/ml | 10 µg/ml | 15 µg/ml | 20 µg/ml | IC50 µg/ml | ||
| 1 | 3a | 20.51 | 35.61 | 79.74 | 86.04 | 10.37 |
| 2 | 3b | 35.89 | 42.67 | 70.25 | 85.44 | 10.78 |
| 3 | 3c | 19.54 | 38.93 | 62.65 | 75.82 | 12.72 |
| 4 | 3d | 28.61 | 39.67 | 59.75 | 86.70 | 11.72 |
| 5 | 3e | 24.45 | 44.76 | 71.51 | 77.84 | 11.56 |
| 6 | 3f | 30.65 | 43.62 | 79.11 | 87.97 | 10.38 |
| 7 | 3g | 28.64 | 39.67 | 73.41 | 90.05 | 10.82 |
| Std. | Ascorbic acid | 38.99 | 55.78 | 72.51 | 93.15 | 9.52 |
Table 2: Antioxidant activity of pyrazoline derivatives (IC50 in µg/mL).
Anti-inflammatory activity
Anti-inflammatory agents inhibit the cyclooxygenase enzymes which are responsible for the conversion of arachidonic acid to prostaglandins. Because Human Red Blood Cell (HRBC) membranes are similar to these lysosomal membrane components, the prevention of hypotonicity induced HRBC membrane lysis was taken as a measure in estimating anti-inflammatory activity. Thus, HRBC membrane stabilization method was used to estimate anti-inflammatory activity of all synthesized compounds.
According to this result, the newly synthesized pyrazolines had more promising anti-inflammatory activity compared with β- diketones and flavones. Compound 3c showed the most promising activity (EC50=11.00 ± 1.26 μg/mL). Among the series. Compounds 3d and 3e showed good anti-inflammatory activity. Compared with standard Aspirin (EC50=11.70 ± 0.98 μg/mL). Fresh whole human blood was collected and it was mixed with equal volumes of sterilized Alsever’s solution (dextrose 2%, sodium citrate 0.8%, citric acid 0.05%, sodium chloride 0.42% and distilled water 100 mL). this blood solution was centrifuged at 3000 rpm for 10 min and was washed three times with equal volume of normal saline. The volume of the blood is measured and reconstituted as 10% v/v suspension with normal saline. The reaction mixture consists of 1.0 mL of test sample of different concentrations in normal saline and 0.5 mL of 10% HRBC suspension, 1 mL of 0.2 M phosphate buffer, 1 mL hyposaline were incubated at 37°C for 30 min and centrifuged at 3000 rpm for 30 mints, the haemoglobin content of the supernatant solution was estimated spectrophotometric ally at 560 nm. Each experiment was performed in triplicate. Dichlorofenac sodium was used as standard and distilled water as control in this study. Where the blood control represents 100% lysis or zero percent stability, the percentage of HRBC haemolysis calculated by formula (Table 3).
% Haemolysis=100-absorbance of sample/absorbance of control × 100
| Compounds | Anti-inflammatory activity in µg/mL (EC50 ± SD) |
|---|---|
| 2a | 58.23 ± 2.36 |
| 2b | 54.00 ± 2.13 |
| 2c | 52.23 ± 2.19 |
| 2d | 36.00 ± 2.03 |
| 3a | 50.13 ± 2.03 |
| 3b | 49.99 ± 1.33 |
| 3c | 11.23 ± 1.26 |
| 3d | 14.03 ± 2.15 |
| 3e | 15.98 ± 0.57 |
| Aspirin | 11.70 ± 0.98 |
Table 3: Anti-inflammatory activity of pyrazoline derivatives (IC50 in µg/mL).
Antimicrobial activity: All the synthesized compounds were screened for their antibacterial Staphylococcus aureus and Escherichia coli organisms using a modified agar diffusion assay method streptomycin was used as positive control. Incubation time and period for antibacterial activity was at 37℃ for 24 hrs results of antimicrobial activities are expressed in terms of ZOI. The result of activity of the synthesized compounds is shown in Table 3 respectively. Based upon the value of ZOI in mm, compounds are classified as, significantly active (ZOI ≥ 15), moderately active (ZOI 13 to 14), poor active (ZOI 10 to 12) and least active (ZOI less than 10). The investigation of antibacterial screening shows that compound 3b exhibited highest activity against E.coli, 3c have maximum zone of inhibition against S. aureus respectively. Overall compound 3a and 3e had good activity against both gram negative and gram positive bacteria (Table 4).
| Microorganisms → |
Zone of inhibition (mm) of compounds | |
|---|---|---|
| Comp. code |
Gram negative E. Coli |
Gram positive S. aureus |
| 3a | 13 mm | 12 mm |
| 3b | 18 mm | 15 mm |
| 3c | 14.5 mm | 9 mm |
| 3d | 10.5 mm | 10.5 mm |
| 3e | 16.5 mm | 11.7 mm |
| Streptomycin | 21 mm | 24 mm |
Table 4: Antimicrobial activity of pyrazoline derivatives.
RESULTS AND DISCUSSION
Microwave assisted synthesis, in general, has a large impact on synthetic organic chemistry compared to traditional processing of organic synthesis. Microwave enhanced organic synthesis saves significant time and very often improves yields. In order to synthesize the chalcones, benzaldehyde was treated with different heteroaryl active methyl compounds under conventional heating method by using ethanol as a solvent in basic condition. It was found that the synthesis of chalcones attempted by conventional method took a longer time (36 h) for completion of reaction with moderate yield (65%-70%). In order to find the best reaction conditions and to obtain chalcones in a short span of time with improved yield, subsequent studies were carried out using microwave irradiation technique under solvent free condition. As visualized, the reaction proceeded smoothly and the chalcones were obtained in excellent yields (82%-92%) within a few minutes (3-5 min). The assigned molecular structures of all pyrazoline were based on spectroscopic analysis (IR, 1H, 13C NMR, and MS) and elemental analysis data. Regarding the structure of pyrazoline from chalcone, the assignment of 3a, was described. The IR spectra of 3a showed characteristic absorption band at 3251 cm1 and 3384 cm1 due to the presence NH and OH groups.
It has been reported that a, b-unsaturated ketone which are attractive due to their electrophilic properties can react with nitrogenous bases to give the corresponding pyrazolines. Thus, a series of pyrazoline derivatives were obtained by refluxing of chalcones (1a-i) with hydrazine hydrate (2a-i) and INH (3a-i) in ethanol in the presence of catalytic amount of glacial acetic acid (scheme 1), but due to partial solubility of it took longer time for completion of reaction (36 h) and afforded the products with low percentage yield (57%-68%). To accelerate the reaction and to improve the yield of products, the microwave irradiation technique was applied. The microwave-assisted reaction in solvent and catalyst-free environment proceeded efficiently and completed within 3-5 min with excellent yield of products (76%-89%). Formation of pyrazolines (3a, 2d, 2h) established on the basis of IR, 1H NMR, and mass spectral analysis. The IR spectrum of 5a showed two broad absorption bands at 3681 cm1 and 3270 cm1 due to the presence of C=O groups of pyrazoline respectively. The absorption band for the carbonyl group was discernible at 1680 cm1. The 1H NMR spectrum showed a broad singlet at δ 8.7 due to the presence of 1H proton. The presence of pyrazoline unit was clearly indicated by the presence of two double doublets at δ 4.01 (2H), 7.16 (H). Further confirmation of the structure was provided by mass spectrometry, which showed molecular ion peak at m/z 346. During the course of reaction for the synthesis of compounds 2a-i and 3a-i, we examined the reaction time by conventional heating method and microwave irradiation method. It was clear that the microwave assisted synthesis of compounds 2a-i and 3a-i were found to be time specific as compared to conventional method, led to the formation of compounds in short time, and overall yield much higher than the conventional route of synthesis (Table 1).
All the synthesized pyrazoline derivatives were screened for antioxidant activity by using DPPH free radical scavenging assay. Ascorbic acid was used as standard for the activity. The results of antioxidant activity showed that few compounds showed considerable antioxidant activity while the 3c and 3g showed good to excellent antioxidant activity (IC50=48.13 and 46.03 μg/ml), remaining synthesized compounds showed poor activity. All the synthesized pyrazoline derivatives were screened for anti-microbial activity shows that compound 2b and 2e exhibited highest activity against E. coli and S.aureus, 2c have maximum zone of inhibition against S. aureus remaining compound had good activity against bothgram negative and gram-positive bacteria. According to this result, the newly synthesized pyrazolines had morepromising anti-inflammatory activity compared with β-diketones and flavones. Compound 3c showed the mostpromising activity (EC50=11.00 ± 1.26 μg/mL) among the series. Compounds 3d and 3e showed good anti-inflammatoryactivity. Compared with standard aspirin (EC50=11.70 ± 0.98 μg/mL).
CONCLUSION
A series of novel heterocyclic derivatives containing pyrazoline ring were derived from chalcones and based on the study activities have been voted for the anti-inflammatory and as biological screening. From the analytical study such as IR, 1H NMR, 13C NMR and mass spectrometry we got to know determination and identification of the chemical composition of synthesized drugs. As synthesized compounds are found to show moderate to good anti-inflammatory activity in appraisal with standards, further modification in the structure of compounds may lead to development of potent anti-inflammatory moiety. Among the series 3b and 3f found to be most potent anti-inflammatory drugs. It can be concluded that presence of electron withdrawing groups may be responsible for showing anti-inflammatory activity.
From the synthesized derivatives it can be resolved that the results of anti-oxidant activity showed moderate to good activity in appraisal with standards. Among all the synthesized compounds 3c and 3g were found to show most potent activity at 30 ppm and 40 ppm whereas compounds 3d and 3f showed moderate activity among tested compounds. Remaining synthesized derivatives showed very poor activity comparing with standard. Antimicrobial activity was performed by using broth dilution method using streptomycin as standard. Compound 2b and 2e were found to be most active compound and remaining compounds 2a, 2c and 2d showed poor activity among tested compounds. Hence, newly synthesized pyrazoline derivatives possess considerable anti-inflammatory, antioxidant and antimicrobial activity. Structure and biological activity relationship of title compound showed that the electron withdrawing group enhanced the activity.
ACKNOWLEDGEMENTS
SMBT college of pharmacy, Dhamangaon, Nashik, MS-422403 for providing research facility.
COMPETING INTEREST
The authors declare that they have no competing interests.
CONSENT FOR PUBLICATION
Not applicable.
FUNDING
No any kind of financial support from national or international agency was received for the present review work.
AVAILABILITY OF DATA AND MATERIALS
The dataset used and analysed during current study available from the corresponding author on reasonable request.
ETHICS APPROVAL AND CONSENT TO PARTICIPATE
Not applicable.
References
- Siddiqui ZN, et al. Green Chem Lett Rev. 2011:8253.
[Crossref]
- Rousell J, et al. Printed in Great Britain. 1986:279-282.
- Saleh HM, et al. Green Org. 2021:1-14.
- Kendall RV, et al. J Org Chem. 1970;35:2246-2248.
- Noroozi-Pesyan N, et al. J Chinese Chem Soc. 2009;56:1018-1027.
- Toda F, et al. Acc Chem Res. 1995;28:480-486.
- Gschwendt M, et al. FEBS Lett. 1994;347:85-89.
[Crossref] [Google Scholar] [PubMed]
- John R, et al. Cancer Lett. 1995;97:33-37.
[Crossref] [Google Scholar] [PubMed]
- Zangade SB, et al. Green Chem Lett Rev. 2013;8253.
- Rothenberg G, et al. J Am Chem Soc. 2001;123:8701-8708.
[Crossref] [Google Scholar] [PubMed]
- Shaik P, et al. Org Chem. 2019:1-12.
- Abrigach F, et al. Der Pharma Chem. 2014;6:1-7.
- Teruna HY, et al. Indones J Chem. 2019;19:583-591.
- Bansal H, et al. Indo Glob J Pharm Sci. 2016;6:01-13.
- Mahavidyalaya E, et al. Asian J Chem. 2007;19:3353-3362.
- Muralidharan V, et al. Int J Pharm Sci. 2018;10:2-7.
- Naraboli BS, et al. Asian J Pharm Clin Res. 2018;11:1-8.
- Bhuiyan M, et al. Chem J. 2011;1:21-28.