Original Articles: 2021 Vol: 13 Issue: 10
Extraction of Oil from Quercus infectoria Galls and Anti-bacterial Properties
Abstract
Quercus infectoria galls were used to extract oil using three solvents namely, acetone 100%, ethanol 99% and distilled water. The oil extraction was made by shaking the samples in the respective solvents for 24 hours and 16 hours in the orbital shaker. Thin Layer Chromatography (TLC) and UV spectrophotometer has been used to separate and quantify the tannic acid in the oil extract of Q. infectoria. From the observation, it was concluded that the oil extract obtained using the acetone solvent has the highest yield (85%) and largest inhibition zone was observed and also tannic acid content was found to be higher in the acetone extracted oil which has been kept for 24 hours. Aqueous, other solvent was also found to have the highest content of tannic acid. None of the oil extracts exhibits any inhibition to E.coli. This finding provides into the usage of oil extract in treatment of skin inflammation caused by bacteria, Propionibacterium acne.
Keywords
Quercus infectoria; Tannic acid; Anti-bacterial activity; Thin layer chromatography
Introduction
Microbes are called disease-causing microbes and can make humans, animals and plants sick by causing infection and disease. Disease-causing microbes can also be called pathogens, germs or bugs and are responsible for causing infectious diseases. Numerous studies have been conducted on the potential of traditional plants on their biologically activity for medicinal purpose [1]. The biological activity of these plants is related to their bioactive compounds which definitely produce physiological actions on human body. The extraction of active compounds is highly depending on the polarity of the solvent because polar compound is easily extracted using polar solvent. Quercus infectoria Olivier (Family: Fagaceae) is a small tree widely distributed in Greece, Asia Minor and Iran. The tree bears galls that emerge on its shoots as a consequence of attack of gall wasp, Cypnis gallae-tincotoriae. The decoction of galls is usually employed as an astringent, gargle and enema [2]. In Asian countries, the galls of Quercus infectoria have been used for centuries in oriental traditional medicines for treating inflammatory diseases. Q. infectoria galls have been widely used as medicinal products which possess antimicrobial and antibacterial activity. Tannin is the main constituents present about 50%-70% of the galls.
Q. infectoria is one of the traditional plants that have great potential on the medicinal purpose and have been reported to possess antioxidant and antibacterial. In Asian, it has been used for centuries as traditional medicine for treating inflammatory disease while in Malaysia it has been used as an herbal drink to treat the women after their childbirth to restore the elasticity of the uterine wall [3]. Other than that, by using the hot water extract of Q. infectoria as a mouth antiseptic, it can control the inflammation of tonsils, while the direct application of it onto the skin cures swelling or inflammation. Moreover, this herb also show promising results in cosmeticeutical where it was reported that the galls possess high potential in skin whitening. The galls is greatly used as medicinal plant since ancient times because it was reported to contain large amount of bioactive constituents such as tannins, gallic acid, syringic acid and others. The main constituents found in the galls of Q. infectoria are tannin (50%-70%) and small amount of free gallic acid and ellagic acid. Most study on Q. infectoria was on identification and isolation, biological and pharmacological studies. However, none of the study reported on the effects of solvents toward antioxidant and antibacterial activity [4].
The galls help overcome bad odours of genital areas. Extract of galls solution is used for washing infected or smelling parts to overcome foul smell emanating from the private areas of women besides terminating bacteria or fungi in these areas. Galls are considered to be the best remedy for vaginal or uterine prolapse, abnormal uterine bleeding [5]. Oral consumption of galls helps in the relief of rectal bleeding. Children suffering from diarrhoea and irritable bowel syndrome or rectal prolapsed may find to relief by gall. Its anti-diarrheal property helps to heal all abdomen related disorder. Galls are used as an excellent wound healer. This antiseptic property is due to the astringent characteristics helps relieve wound faster.
The objective of this study is to extract oil Q. infectoria galls using three different solvents, to identify and quantify tannic acid using TLC and UV Spectrophotometer and to evaluate the antibacterial activity of different extracts of oil [6].
Materials and Methods
The plant material Quercus infectoria galls were collected from local herbal shop in Coimbatore, South India.
Chemicals and reagents used are tannic acid, acetone (100%), ethanol (99%), gelatin, ferric chloride, sodium chloride was purchased from analytical grade [7-9].
Micro-organisms gram positive bacteria such as Staphylococcus aureus, Bacillus subtilis, Propionibacterium acne (collected from microbial type culture collection) and gram negative bacteria such as Escherichia coli and Klebsiella pneumoniae.
Extraction of oil from plant material
To prepare the extract, 10 grams of powdered gall was dissolved in 100 ml of three solvents namely acetone (100%), ethanol (99%) and water and placed in orbital shaker. Two sets of extraction process were done: one by shaking the samples (gall powder) in the respective solvents for 24 hours and second for 16 hours in the shaker at 100 rpm [10]. After the respective times, the mixture was filtered using Whattman filter paper 6 mm and the filtrate was transferred to a round-bottomed flask and concentrated in the rotary vacuum evaporator to remove the solvent. Rotary evaporator or “rotovaps” may be found wherever processes require sample concentration or solvent distillation. Solvents were removed from the filtrate with the respective boiling point of three solvents. After the removal of solvents, the oil extracted was collected and stored in an air tight container.
Characterisation of Quercus infectoria tannic acid:Q. infectoria galls were used to determine the tannic acid. Air-dried samples (galls) were ground. In order to determine the samples, it was characterised by TLC, UV and chemical test and was compared with the reference (standard) tannic acid [11]. Tannic acid, a specific form of tannin, a type of water soluble phenolic compounds with the molecular weight between 500 and 3000 and have the special properties such as the ability to precipitate alkaloids, gelatine and other proteins. Tannic acid is also called as acidum tannicum, gallo-tannic acid, gallo tannin, tannimum, quercotannin acid, querci-tannic acid and qurecotannic acid. Its weak acidity is due to numerous phenol groups. The chemical formula for tannic acid is C76H52O46. Commercial form of tannic acid is extracted from any part of the plants: Tara pods, gallnuts from Quercus infectoria or Sicilian Sumac leaves (Rhus coriaria). Tannic acid is a spongy, brilliant, light, odourless white, or commonly yellowish, solid [12]. It dissolves in water, alcohol, and ether, but less so in ether than in alcohol. In the solid state it is unalterable in the air but, dissolved in water, it absorbs oxygen, and is transformed into carbonic acid, which escapes, and gallic acid, which remains in solution: hence it should be dissolved only at the time we are about to use it. Tannins are divided into three main classes. The condensed tannins (proanthocyanidins) are flavanol based biopolymers that at high temperature in alcohol solutions of strong mineral acid release anthocyanidins and catechins as end groups. Gallo tannins and ellagitannins belong to hydrolysable tannins.
Separation of tannic acid by TLC: The oil obtained from the extraction process was used for the separation of tannic acid by Thin Layer Chromatography (TLC) [13]. Aluminium coated TLC plates of size 8.5 cm length and 2.5 cm width was used. Acetic acid and chloroform were used as developing solvent in the ratio of 1:9. The oil extract was dissolved in acetone, ethanol and water and separated by TLC and compared with the standard tannic acid. Developing chromatogram-separation of phenolic compound: A concentrated sample solution of the phenolic was mixed in the respective solvents. Spot two samples, one the oil extract and second the standard phenol called tannic acid was placed on the TLC plate. The loaded plates were placed inside TLC chamber which is pre-saturated mobile phase (acetic acid and chloroform). The elution was allowed to reach to 7-8 cm length. Take out the plate from the chamber and allow it to dry at room temperature. After separation/elution, the solvent moved and solute moved in cm was marked using pencil [14-16].
Analysis and calculation
Retardation factor (Rf) value was calculated using the formula
Rf =Distance travelled by the solute (cm)/Distance travelled by the solvent (cm)
UV spectrophotometry of total phenols
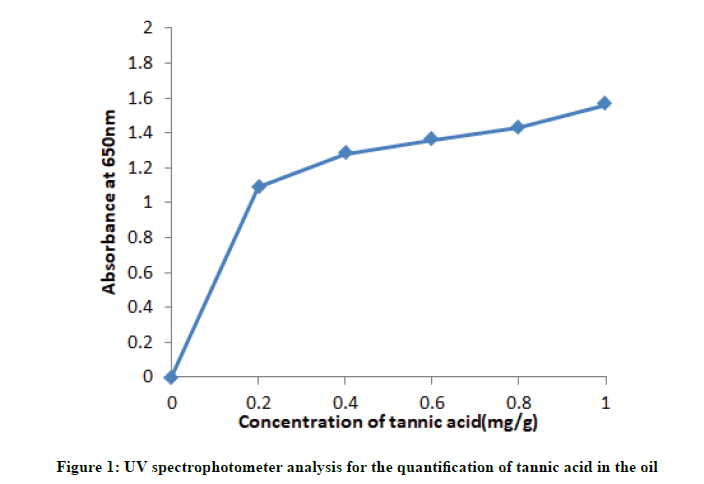
Quantitative estimation of total phenols was done by Folin Ciocalteu Reagent (FCR) method. Phenols react with phosphor molybdic acid in Folin Ciocalteu Reagent (FCR) in alkaline medium and produce blue coloured complex called as molybdenum blue. Phenols in each samples was estimated by the following procedure: Dissolve 100 mg of standard tannic acid in 100 ml of distilled water and 10 ml of stock was dissolved in 100 ml of distilled water for a working solution. Approximately 0.1 ml of oil sample was dissolved in respective solvent and made upto 1 ml with distilled water [17]. This 0.5 ml Folin Ciocalteu Reagent (FCR) was added (diluted 1:10 v/v). After 5 minutes 2 ml of 20% sodium carbonate solution was added. Incubate the mixture for 10 minutes at room temperature. Absorbance was measured against the blank at 650 nm using UV spectrophotometer. A calibration curve was constructed using tannic acid and total phenol content of the extract was expressed in terms of milligrams of tannic acid per gram of dry weight.
Phytochemical screening
i) Test with ferric chloride solution: To 1 ml of each oil extract was taken in a test tube and few drops of ferric chloride (1%) solution was added. A green or black precipitate appears which indicates the presence of tannic acid [18].
(ii) Test with gelatin and NaCl solution: To 1 ml of each oil extract, 1 ml of gelatine and NaCl (1%) solution was added. A white precipitate was observed which shows the presence of tannic acid.
(iii) Detection of flavonoids: Oil was treated with few drops of lead acetate solution. Formation of yellow colour precipitate indicates the presence of flavonoids [19].
(iv) Detection of alkaloids:
Mayer’s test: Oil treated with Mayer’s reagent (potassium mercuric iodide). Formation of yellow precipitate indicates the presence of alkaloids [20].
Dragendroff’s test: Oil was treated with Dragendroff’s reagent (solution of Potassium Bismuth Iodide). Formation of red precipitate indicates the presence of alkaloids.
Anti-bacterial assay
The well diffusion method was used to evaluate the antibacterial activity of oil extract according to Basri 2012. Muller Hinton agar was used as a media for the test microorganisms [21]. The oil extract from the galls of Quercus infectoria were screened against gram positive bacteria such as Staphylococcus aureus, Bacillus subtilis and Propioni bacterium acne and gram negative bacteria such as Escherichia coli and Klebsiella pneumonia. First, the 50 ul of bacterial suspension was applied on the agar plates and swabbed throughout the plate using sterile hockey stick. After which the well was made in the agar plate using well puncher and impregnated with 20 ul of each of oil extract. Then, streptomycin disc was used as a standard to confirm the microorganism tested was inhibited by antibiotic. All the plates were incubated for 24 hours at 370°C. Then the antibacterial activity of oil was interpreted from the size of diameter of zone of inhibition to the nearest inhibition as observed from the clear zone surrounding the well. The inhibition zone was measured after 24 hours [22].
Results and Discussion
Solvent effect on oil extraction yield
Based on Table 1, the highest extraction of oil yield was found with acetone extracted oil (85%) from 24 hours and with slight difference followed by water extracted oil (80%) from 24 hours, ethanol extracted oil (70%). On the other hand 99% ethanol which is used for the extraction of oil for 16 hours resulted on the lower extraction yield. From the above Table 1, the purity percentage of tannic acid was also calculated, in which the acetone extracted oil from 24 hours contains the highest amount of tannic acid (69%) and followed by water. A polar solvents yield polar compounds and non-polar solvents will extract non-polar compounds thus different solvents will yield different extracts. The suitable solvents for extracting target compound will be based on the type of solvents used [23]. However, ethanol and water are widely used solvents due to their low toxicity and high extraction yield and in advances their polarity can modulated by mixing them at selected ratio [24].
| Types of solvent | Yield of oil (%) | Density of oil (g/cm3) | Purity of tannic acid (%) |
|---|---|---|---|
| Acetone (24 hrs) | 85 | 1.46 | 69 |
| Ethanol (24 hrs) | 70 | 1.02 | 48.2 |
| Aqueous (24 hrs) | 80 | 1.05 | 50.47 |
| Acetone (16 hrs) | 72 | 1.03 | 48.57 |
| Ethanol (16 hrs) | 52 | 1 | 47.27 |
| Aqueous (16 hrs) | 70 | 1.01 | 52.4 |
Table 1: Total oil extraction yield from Quercus insectoria using different types of solvents.
Thin Layer Chromatography (TLC): TLC is a solid liquid-analytes separation technique for assaying the purity of organic compounds. TLC requires only few Nano grams of sample for an analysis. TLC takes the advantages of the different affinity of the analytes with the mobile phase and stationary phase to achieve separation of complex mixtures of organic molecules. From Table 2, it is clear that the tannic acid is separated and the retention factor for the oil extract and standard tannic acid was found to be equal for all the three types of solvent.
| Solvents | Retention factor for 24 hrs oil extract | Retention factor for 16 hrs oil extract | Retention factor for original tannic acid |
|---|---|---|---|
| Acetone | 0.11 | 0.17 | 0.18 |
| Ethanol | 0.062 | 0.1 | 0.08 |
| Aqueous | 0.08 | 0.12 | 0.1 |
Table 2: TLC of standard tannic acid and oil from Q. infectoria galls
UV spectrophotometry of tannic acid: The UV absorption spectra of the oil extract and standards, they have shown that acetone and ethanol extracted oil of 24 hours contains the highest amount of tannic acid and also indicates the presence of tannic acid. From Table 3 it is clear that the concentration of tannic acid was found to be high in acetone and ethanol extracted oil (0.66 and 0.55 mg/g) of 24 hours extract, while in aqueous oil extract it was found to be 0.43 mg/g. Similarly, in 16 hours extract concentration of tannic acid were higher in acetone and ethanol (0.64 and 0.54 mg/g) and in aqueous extracted oil it was 0.42 mg/g (Figure 1).
| Name of standard/sample | Absorbance at 650 nm | Concentration of tannic acid (mg/g) |
|---|---|---|
| Tannic acid | 1.09 | 0.2 |
| Tannic acid | 1.28 | 0.4 |
| Tannic acid | 1.36 | 0.6 |
| Tannic acid | 1.43 | 0.8 |
| Tannic acid | 1.56 | 1 |
| Oil extracted using acetone (24 hrs) | 1.42 | 0.66 |
| Oil extracted using ethanol (24 hrs) | 1.22 | 0.55 |
| Oil extracted using aqueous (24 hrs) | 1.01 | 0.43 |
| Oil extracted using acetone (16 hrs) | 1.19 | 0.64 |
| Oil extracted using ethanol (16 hrs) | 1.38 | 0.54 |
| Oil extracted using aqueous (16 hrs) | 0.97 | 0.42 |
Table 3: UV spectrophotometer analysis of concentration of tannic acid (mg/g) in each of the Q. infectoria gall oil
Phytochemical screening: Phytochemical screening was done to identify the presence of tannic acid, flavonoids, and alkaloids using ferric chloride, gelatine, sodium chloride, lead acetate, Mayer’s reagent and Dragondroff’s reagent (Table 4).
| Property of test | Acetone extracted oil (24 and 16 hrs) | Ethanol extracted oil (24 and 16 hrs) | Aqueous extracted oil (24 and 16 hrs) |
|---|---|---|---|
| Ferric chloride | Positive | Positive | Positive |
| Gelatin and NaCl | Positive | Positive | Positive |
| Lead acetate (Flavonoids) | Positive | Positive | Positive |
| Mayer’s reagent (Alkaloids) | Negative | Negative | Negative |
| Dragendroff’s reagent (Alkaloids) | Negative | Negative | Negative |
Table 4: Chemical tests for the Q. infectoria gall oil
Antibacterial assay: As can be seen from Tables 5 and 6, all the gall oil showed inhibitory effects against bacterial species tested. After 24 hours, the largest inhibition zone was shown by the oil extract of 100% acetone (33.00 mm) against Staphylococcus aureus and Bacillus subtilis (30.0 mm). However B. subtilis was found to be most susceptible towards all of the oil extracts [24]. While, E.coli was found to be not susceptible to the oil extract. Also the standard tannic acid was also tested against the bacterial species which also resulted in the positive inhibition zone. This finding was in accordance with previous research which had reported that the plant extract using organic solvent exhibited more antibacterial activity compared to the extract using aqueous as a solvent (Figure 2).
| Microorganism | Inhibition zone of tannic acid (mm) | Inhibition zone of acetone extracted oil (mm) | Inhibition zone of ethanol extracted oil (mm) | Inhibition zone of water extracted oil (mm) |
|---|---|---|---|---|
| Bacillus subtilis | 25 | 30 | 30 | 29 |
| Propinibacterium acne | 20 | 26 | 28 | 24 |
| Staphylococcus aureus | 23 | 24 | 21.5 | 20 |
| Klebsiella pneumonia | 19 | 18 | 17 | 17 |
| Escherichia coli | - | - | - | - |
Table 5: Antibacterial activity of 16 hours Q. infectoria gall oil
| Micro-organisms | Inhibition zone of tannic acid (mm) | Inhibition zone of acetone extracted oil (mm) | Inhibition zone of ethanol extracted oil (mm) | Inhibition zone of water extracted oil (mm) |
|---|---|---|---|---|
| Bacillus subtilis | 25 | 23 | 21 | 27 |
| Propinibacterium acne | 20 | 28 | 27 | 23 |
| Staphylococcus aureus | 23 | 33 | 28 | 24 |
| Klebsiella pneumonia | 19 | 20 | 18 | 19 |
| Escherichia coli | - | - | - | - |
Table 6: Anti-bacterial activity of 24 hours Q. infectoria gall oil
Further this present study also showed that the oil extracts from the galls were active in both gram positive and gram negative bacteria [25]. In addition, the most interesting finding was that inhibition zone showed by all the extracts was bigger compared to the positive control against E. coli. The antibacterial activity of the oil extract may be due to the presence of tannic acid.
Conclusion
This study has shown that the extraction of oil from Quercus infectoria galls using three solvents namely, acetone 100%, ethanol 99% and distilled water has been successfully made. The oil extraction was made by shaking the samples in the respective solvents for 24 hours and 16 hours in the orbital shaker. Result showed that 100% acetone was the most efficient solvent among the others. Acetone extracted oil was the highest yield where 10 g of gall powder in 100 ml of solvent gave the final yield of 8.5 g of oil. The experiment results showed that the conditions followed during the extraction had a great impact on the extractability of oil and total phenol (tannic acid) from the Q. infectoria galls. Generally the combination of acetone and water seem to be more effective when compared with methanol and ethanol. Thin Layer Chromatography and UV spectrophotometer has been separated and quantified the tannic acid in the oil extract of Q. infectoria. The strong antibacterial activities of Q.infectoria are probably due to the bioactive compounds present in the plants. This finding provides into the usage of oil extract in treatment of skin inflammation caused by bacteria, Propionibacterium acne. It also helps to stop the dysentery caused by Bacillus subtilis [26].
Acknowledgment
The author gratefully acknowledges Karunya University, Tamilnadu, India for providing the necessary help for carrying out the present work. Also would like to thanks to my friends support to complete to this work.
References
- Mohemmadi Z. Int J Pharma Bio Sci. 2011; 2(1): 609-615.
- Goli AH, Barzegar M, Sahari MA. Food Chem. 2004; 92: 521-525.
- Samuelson G. J Pharm. 1992; 79: 86.
- Nadkarni AK. Ind Met Med. 1982; 1: 1042-1043.
- CSIR. Ind Pro. 1995; 53: 351.
- Kaur G, Hamid H, Ali A, et al. J Ethno Pharma. 2004; 90(3): 285-292.
- AL-Ghanimi AA, AL-Ethari AY, Abdulhusain HK. J Pharm. 2007; 5(4): 227-234
- Everest A, Ozturk E. J Ethno Med. 2005; 1: 1-6.
- Hamid H, Kaur G, Abdullah ST, et al. Pharma Bio. 2005; 43: 317-323.
- Galla BP. Pharma Soc Britain. 1911.
- Kaur G, Hamid H, Ali A, et al. J Eth Pharma. 2004; 90(3): 285-292.
- Chopra RN, Nayar SI. Chopra IC. Plant Med. 1956; 57: 208.
- Rohana S, Vimala S, Abdull Rashih A, et al. Plant Med. 2004.
- Dar MS, Ikram M, Fakouhi T. J Pharma Sci. 1976; 65: 1791-1794.
- Ikram M, Nowshad F. Plant Med. 1977; 31: 286-287.
- wang JK, Kong TW, Baek NI, et al. Plant Med. 2000; 66: 273-274.
- Evans WC. Pharmcopia. 1996.
- Wiart C. Kumar A. J Pharm. 2001.
- Basri DF, Fan SH. Ind J Pharm. 2005; 37: 26-29.
- Hamid H, Kaur G, Abdullah ST, et al. Pharma Bio. 2005; 43: 317-323.
- Asghari J, Ondruschka B, Mazaheritehrani M. J Med Plant Res. 2011; 5(4): 495-506.
- Karamac M, Kosinska A, Rybarczyk A, et al. Polsh J Food Nutri Sci. 2007; 57(5): 471-474.
- Slinkard K, Singleton VL. Am J Enol Vitic. 1977; 28: 49-55.
- Zarnowski R, Suzuki Y. J Food Comp Anal. 2004; 17: 649-656.
- Franco D, Sineiroz J, Rubilar M, et al. Elec J Env Agri Food Chem. 2008; 7(8): 3210-3216.
- Parekh J, Jadeja D, Hanlin R. Turkish J Bio. 2005; 29: 203-210.