Original Articles: 2021 Vol: 13 Issue: 2
Development and Evaluation of 1, 3 Dibromo Propane as a Genotoxic Impurity in Olopatadine Hydrochloride Drug Substance by Using HSGC
Vinayak T. Vele*, Krupali Shah, Vishal Telvekar, Rupesh Kelaskar and Mohan A Chandavarkar
R & D Synthetic API Analytical Development Laboratory, FDC Ltd., 142-148, S.V. Road, Jogeshwari (W), Mumbai-400102, Maharashtra, India
Abstract
A simple and accurate gas chromatography head space method was developed for the determination of 1, 3 Dibromo Propane in the Olopatadine Hydrochloride bulk drug. Chromatographic separation between 1, 3 Dibromo Propane, diluent and residual solvents used in Olopatadine Hydrochloride was achieved using a DB-624 (30 m x 0.53 mm x 3.0 μm) column using nitrogen as carrier gas. The resolution between the between 1, 3 Dibromo Propane and Benzyl alcohol as diluent was found to be more than 5.0. The Limit of Detection (LOD) and limit of quantification (LOQ) of the 1, 3 Dibromo Propane was 1.46 and 4.37 μg mL-1, respectively. The percentage recoveries of the 1, 3 Dibromo Propane ranged from 95.1% to 111.5 in the samples of Olopatadine Hydrochloride. The developed method was validated as per International Conference on Harmonization guidelines in terms of specificity, limit of detection, limit of quantification, precision, linearity, accuracy and ruggedness.
Keywords
Development; Validation; Olopatadine hydrochloride; 1, 3 Dibromo propane; Genotoxic; HSGC
Introduction
Olopatadine Hydrochloride [1,2] (trade name Patanol), 2-[(11Z)-11-[3-(dimethylamino)propylidene]-6H-benzo[c][1]benzoxepin-2-yl]acetic acid; hydrochloride (Figure 1), is a medication that used to treat itching of the eye caused by a condition known as allergic conjunctivitis (pink eye) [2]. It works by preventing the effects of certain inflammatory substances, which are produced by cells in your eyes and sometimes cause allergic reactions [2,3].1,3 Dibromo Propane was one of the key raw material used in the manufacturing process Olopatadine Hydrochloride. 1, 3 Dibromo Propane is halogenated hydrocarbons having the structural alert for the genotoxicity [4,5].
The study is proposed and conducted for the method development and further validation of method for determination of 1, 3 Dibromo Propane in Olopatadine Hydrochloride drug substance. Recommended maximum daily dose for Olopatadine Hydrochloride is 5.32 mg. Based on the genotoxic impurity guideline, daily dose and threshold of toxilogical concern (TTC) approach, the limit for the 1, 3 Dibromo Propane is decided as 282 μg/mL [5,6,7]. In the present work we have developed a simple precise method for determination of 1, 3 Dibromo Propane in Olopatadine Hydrochloride using Capillary column by Head space Gas chromatography. The developed method was validated according to International Conference on Harmonization (ICH) guidelines [8].
Experimental Section
Chemicals and Reagents
Samples of Olopatadine Hydrochloride were obtained from R & D synthetic department of FDC Ltd, Mumbai, India. HSGC-grade Benzyl alcohol was procured from Merck, Darmstadt, Germany. 1, 3 Dibromo Propane standard procured from Loba Chemie/Rankem.
Instrumentation
Gas chromatography system used was Perkin Elmer (Clarus 600 series, US) system equipped with Head space sampler turbo matrix 110 and Flame Ionisation Detector (FID). The output signal was monitored and processed using Chromeleon software 6.8.
Chromatographic Condition
The chromatographic column used was capillary DB-624 column (30 m × 0.53 mm × 3.0 μm), (Agilent, USA). The carrier gas used was Nitrogen. The Air and Hydrogen gases were used with flow rate of 400 ml/minute and 40 ml/minute. The Oven program starts with initial temperature 100°C hold for 5 minutes. Further ramp in temperature by 10°C/minute up to 240°C with hold for 5 minutes. The flow rate of carrier gas was 2.0 mL/min. The injector and detector column temperature was maintained at 220°C and 240°C respectively. The Split Ratio, range and attenuation was set as 2:1, 1 and 1 respectively. The headspace parameters was set having Vial Oven Temperature 100°C, Needle Temperature 110°C and Transfer line temperature 120°C. The Thermostat time and GC cycle time was set as 30 minutes and 35 minutes respectively. The injection volume used was 1.2 mL. Benzyl alcohol was used as diluent.
Preparation of Standard Solutions
The stock solution of the 1, 3 Dibromo Propane were prepared by dissolving an appropriate amount of the standard in diluent. For quantitation of 1, 3 Dibromo Propane in Olopatadine Hydrochloride a solution of 14.1 μg/mL concentration was used. The target analyte concentration was fixed as 50.0 mg mL-1.
Results and Discussion
Method Development
A solution of Olopatadine Hydrochloride and 1, 3 Dibromo Propane (50 mg/mL and 14.1 μg/mL) prepared in diluents for method establishment. To develop a rugged and suitable GCHS method for the separation, different stationary phases and temperature programmed were employed. Preliminary column screening involved different types of capillary column such as DB-1, DB-5, OV-17 and DB-624 were employed. On DB-624 column provided selectivity between the all the process solvents used in the manufacturing process of Olopatadine Hydrochloride and 1, 3 Dibromo Propane peak using Nitrogen as carrier gas. Good separation was achieved on DB-624 column with temperature programed.
Optimized Chromatographic Conditions
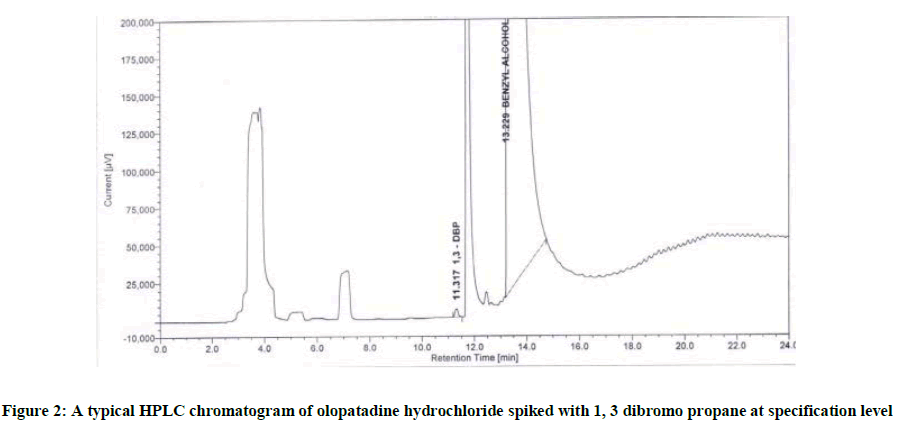
Due to the better chromatographic results obtained on the DB-624 column, further method optimization and quantification of the 1, 3 Dibromo Propane were carried out on this column. Based on the data obtained from method development and optimization activities, the DB-624 column (30 m × 0.53 mm × 3.0 μm) column with the Nitrogen as carrier gas with temperature programmed was selected for the final method. In the optimized method, the typical retention times of the Diluent (Benzyl alcohol) and 1, 3 Dibromo Propane were approximately 14.10 and 12.15 min, respectively. Baseline separation between diluent, solvents used in the manufacturing process of Olopatadine Hydrochloride and 1, 3 Dibromo Propane was obtained with a total run time of 24 min. The system suitability results were given in Table 1.
| Component | Retention time (min) | Relative retention time (min) | Resolution | Tailing factor |
|---|---|---|---|---|
| 1, 3 Dibromo Propane | 12.15 | 0.86 | -- | 0.95 |
| Benzyl alcohol | 14.10 | 1.00 | 6.2 | --- |
Table 1: System suitability criteria
The structure of 1, 3 Dibromo Propane and Olopatadine Hydrochloride are displayed in Figure 1. The typical chromatogram of the Olopatadine Hydrochloride spiked with 1, 3 Dibromo Propane displayed in Figure 2.
Method Validation
Precision: The precision of an analytical procedure expresses the closeness of agreement among a series of measurements obtained from multiple samplings of the same homogenous sample under prescribed conditions. The system and method precision for the 1, 3 Dibromo Propane was checked at its specification level (i.e. 14.1 μg/ml with respect to analyte concentration, 50.0 mg mL-1). The percentage RSD of method repeatability and system repeatability for the 1, 3 Dibromo Propane were found to be 2.75% and 1.67% respectively, which confirms good precision of the method.
Linearity: The linearity of an analytical procedure is its ability (within a given range) to obtain test results, which are directly proportional to the concentration of the analyte in the sample. The linearity of the method for the 1, 3 Dibromo Propane was checked at six concentration levels, i.e., from limit of quantitation (30%), (50%) to 150% of the 1, 3 Dibromo Propane specification level (14.1 μg/ml), which is with respect to of Olopatadine Hydrochloride test concentration. The coefficient of regression of the calibration curve was found to be 0.9926, thus confirming the excellent correlation between the peak area and concentration of the 1, 3 Dibromo Propane.
Limit of detection and limit of quantitation: The limit of detection (LOD) and limit of quantification were achieved by injecting a series of dilutions of 1, 3 Dibromo Propane [8]. The precision of the developed method for 1, 3 Dibromo Propane at LOD and LOQ was checked by analysing standard solutions prepared at the LOD and LOQ level and calculating the percentage relative standard deviation of area. The limit of detection and quantification for 1, 3 Dibromo Propane was found to be 1.46 μg mL-1 and 4.37 μg mL-1 respectively.
Ruggedness and robustness: The ruggedness [8] of a method was defined as degree of reproducibility of results obtained by analysis of the same sample under a variety of normal test conditions such as different analysts, different instruments and different days. The recovery experiments carried out for the 1, 3 Dibromo Propane in Olopatadine Hydrochloride samples at the same concentration levels tested. The data obtained from both the experiment was well in agreement with each other, thus proving the method ruggedness. The robustness [8] of an analytical procedure is measured by its capability to remain unaffected through small, but deliberate, variations in method parameters and provide an indication of its reliability during normal usage. In the varied chromatographic conditions like Carrier gas flow, Oven Temperature and HS Temperature, the resolution between the peaks of 1, 3 Dibromo Propane and Olopatadine Hydrochloride was found to be >5.0 illustrating the robustness of the method.
Recovery of 1, 3 dibromo propane: The standard addition and recovery experiments were conducted for the 1, 3 Dibromo Propane in bulk samples of Olopatadine Hydrochloride in triplicate at LOQ (4.37 μg/mL), 50% (7.05 μg/mL), 100% (14.1 μg/mL) and 150% (21.1 μg/mL) with respect to test concentration. The percentage recovery ranged from 95.1% to 111.5% (Table 2).
| Parameter | µg mL-1 | r | %Mean recovery | %RSD |
|---|---|---|---|---|
| LOD | 1.46 | - | - | 12.9 |
| LOQ | 4.37 | - | - | 6.24 |
| Linearity | ||||
| (LOQ to 150%) | - | 0.9926 | - | - |
| Accuracy | ||||
| LOQ% spiking | - | - | 111.5 | 3.25 |
| 50% spiking | - | - | 103.2 | 2.16 |
| 100% spiking | - | - | 95.1 | 0.97 |
| 150% spiking | - | - | 96 | 1.03 |
| Precision | ||||
| System precision | - | - | - | 1.67 |
| Method precision | - | - | - | 2.75 |
| Intermediate pre (Ruggedness) | - | - | - | 5.05 |
Table 2: Summary of method validation data
Conclusion
A simple, rapid and accurate gas chromatography head space method Chromatography (HSGC) method developed in ordered to separate 1, 3 Dibromo Propane, diluent and residual solvents used in the manufacturing process Olopatadine Hydrochloride. Method validation was carried out using a DB-624 column due to the better chromatographic results achieved on the column. The validated method was demonstrated to be specific, accurate,precise, sensitive and rugged. The developed and validated method can be implemented for the determination and quantitative of 1, 3 Dibromo Propane in Olopatadine Hydrochloride bulk drug.
Acknowledgments
The authors wish to thank the management of FDC’s group for supporting this research work. Authors wish to acknowledge the R & D synthetic group for providing the samples for our research.
References
- Olopatadine.Wikipedia.
- Olopatadine.rxlist.2021
- US pharmacopeia. 2017, 41, 2101
- IARC Monographs on the Evaluation of Carcinogenic Risks to Humans. 2000, 77.
- Assessment and Control of DNA reactive (Mutagenic) impurities in pharmaceuticals to limit of potential carcinogenic risk M7 (R1), 2017
- Guideline on the Limits of Genotoxic Impurities, Committee for medicinal products for human use (CHMP), European Medicines Agency (EMEA), 2006
- US Food and Drug Administration CDER Guidance for industry, 2009
- ICH Validation of analytical procedures: text and methodology Q2 (R1), International Conference on Harmonization, 2005