Original Articles: 2025 Vol: 17 Issue: 2
Copper Phosphate Crystalline Phases in X-ray Diffraction
Aryashree Poudyal Kharel*,
Department of Chemistry, Stanford University, Stanford, United States
*Corresponding Author:
Received: 08-Apr-2024, Manuscript No. JOCPR-24-131719; Editor assigned: 11-Apr-2024, PreQC No. JOCPR-24-131719 (PQ); Reviewed: 25-Apr-2024, QC No. JOCPR-24-131719; Revised: 08-Jan-2025, Manuscript No. JOCPR-24-131719 (R); Published: 15-Jan-2025, DOI:10.37532/0975-7384.2025.17(2).230
Copyright: © 2025 Kharel AP. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
X-ray Diffraction (XRD) spectra and thermogravimetric instruments can be used to analyze the changes in a copper phosphate compound before and after heating. The product synthesized in optimum condition has been subjected first to thermogravimetric analysis and the residue left over was used for X-ray diffraction confirms the presence of copper pyrophosphate in Argon and air as the two different environments at 600°C. The sample prepared from copper dihydrogen phosphate was studied through thermogravimetric analysis and the phases in which the compound existed were confirmed through X-ray diffraction. The copper phosphate compounds were studied by thermal analysis in two different environments, argon and air and on two different surfaces crucible and tungsten disc. Here the copper phosphate compounds decomposition behavior of the mixture and the resulting products were studied through pyrolysis when heated to high temperatures. X-ray diffraction was used to identify the phases formed during decomposition and to study any changes in the crystal structure. By observing the positions and intensities of the diffraction peaks in the spectrum, the crystal structure of the compound was determined along with the phases present. The diffraction pattern is novel to the crystal lattice arrangement, helping us in the identification of different crystalline phases around 100°C-150°C to be orthophosphate and at second transition temperature 200°C-600°C there is the formation of copper pyrophosphate. The diffraction pattern is unique to the crystal lattice arrangement, in this journal I have accurately determined the crystal structure at 600°C for different concentration of copper phosphate compounds to be reported to be copper pyrophosphate at 600°C on different substrate of Tungsten and crucible however the sample prepared of copper phosphate compound before heating was quite ambiguous.
Keywords
X-ray diffraction; Copper phosphate compound; Crystalline phases; Copper pyrophosphate
INTRODUCTION
State and crystal structures of Phosphate of Ca, Sr, Pe have been studied under high pressure for copper phosphate compounds in Xray diffraction have been studied in the past. Copper phosphate chloride with a disordered guest structures crystal chemistry and magnetic property. Novel copper phosphate chloride crystal structure was established based on the low temperature but none of the study has been done at 600°C (high temperature). The interest in these copper phosphate compounds is often used in catalyst or as a precursor for the synthesis of functional material showing the significance of copper in bimetallic palladium copper catalyst improve selectivity in oxygenate coupling reactions as heterogenous catalyst [1].
Williams and Piepmeier in 1972 were the first to investigate the use of a tungsten filament extracted from a light bulb as the atomizer for atomic absorption spectrometry. In ETAAS different kinds of metal have been used as the atomizer. Among them tungsten has become popular due to its various application properties. Tungsten is very stable and has low reactivity with high resistance to concentrated acids. The most important property is its ability to retain its strength at very high temperatures [2].
Copper nitrate can exist in several hydrated forms and proceeds through several stages of thermal decomposition as found out by Boris L’Vov. Wang et al., later used Evolved Gas Analysis and Quadrupole mass analyzer to study the atomization of copper nitrate in a graphite furnace in vacuo. Thermal decomposition of Cu (NO3)2. 3H2O in vacuo proceeds in three stages; the rate maxima lying at about 400, 460 and 530 K. The general scheme of three stage thermal decomposition of Cu (NO3)2. 3H2O to CuO has been refined based on the evolved gas [3].
In 1990 Krakovska proposed a theory of atomization to explain the different types of reactions that happen when a tungsten atomizer is used. There are three basic reaction types of reactions that occur upon atomization from a tungsten surface. First is the evaporation or sublimation of metallic analyte [4].
M(s) → M (l) → M (g)
M(s) → M (g)
MO(s,l,g) + H2(g) → M(s,l,g) +H2O (g)
The transformation of the copper phosphate compounds during heating up to 600°C was studied by M. Watawabe. The decomposition processed through several steps. First is the loss of water of crystallization [5].
M4P2O7.nH2O →M4P2O7. (n-x) H2O + xH2O
(When the products were heated, decomposition of the diphosphate to orthophosphate took place using a fraction of crystallization of water below about 150°C [6].
(n+1)M4P2O7 + (n+1) H2O → 2(n+1)M2HPO4
Copper phosphate compound have it’s importance in thermal insulation, ceramic reinforcement and thermal conductivities, aerospace, automotive, construction was explained by Zizhang Zhan et al [7].
Copper phosphate nanoparticles crystalline nature of the sample was confirmed by X-ray diffraction showing bands of tetragonal distortion around Cu2+ which was proved by Tem analysis was studied by Ramesh and et al [8].
MATERIALS AND METHODS
Sample preparation
Two Cu2+: P2O74- molar concentrations of 1, 0.1 were prepared. The synthesis of copper phosphate compound was carried out using copper nitrate (Fischer Scientific lot # 914413, certified ACS, CAS# 19004-19-4) and tetra sodium pyrophosphate (practical grade No P-8010). The precipitate obtained was light blue and was dried in open air which results in the preparation of copper phosphate compound [9].
The preparation of 1 M copper phosphate species was done using 0.882 g of sodium pyrophosphate in 10 ml deionized water to the 1.544 g of copper nitrate in 10 ml deionized water separately in beakers. A light blue colored precipitate was obtained through gravity filtration. The greatest percentage yield obtained was 55.6% [10].
The second molar concentration of 0.1 M was synthesized by mixing 0.4651 g of copper nitrate in 10 ml deionized water to 0.26470 g of sodium pyrophosphate in 10 ml deionized water separately then the solution was mixed forming a light blue precipitate of copper phosphate compound was recovered through gravity filtration. The yield obtained was 48.9%. The copper phosphate species was synthesized by mixing copper nitrate (Fischer Scientific lot # 914413, certified ACS, CAS# 19004-19-4). Both the solutions were mixed and copper dihydrogen phosphate was formed and then put in a 60°C oven for drying. The sample obtained was the dry blue copper phosphate compound [11].
Instrument
Thermogravimetric Analysis (TGA): The first instrument used was TGA which can provide quantitative information about chemical phenomena including dehydration, decomposition and solid gas reactions. It helps in material characterization through analysis of characteristic decomposition patterns. The thermal decomposition of the analyte was carried out using a Mettler Toledo, TGA/DSC 1 optimum. The instrument can hold a highest temperature up to 1600°C. The sample to be studied is placed in the sample holder or crucible. First the crucible was soaked in nitric acid (0.2% nitric acid) and then rinsed in deionized water overnight. To avoid any water within the crucible it was dried in a 60°C oven, then transferred to a much hotter oven at 1000°C for 20 minutes and cooled overnight.
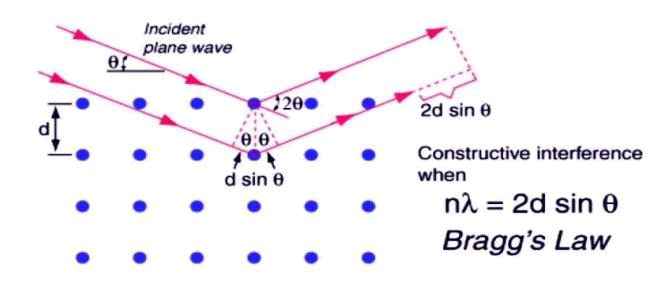
The purpose of the X-ray diffractometer is to analyze the dimensions of a crystalline lattice, determine the structure of a crystalline lattice and compare it to spectra for crystal identification. When a monochromatic beam of X-ray photon falls onto a given specimen three basic phenomena may result: Scattering, absorption and fluorescence. The regular arrangement of atoms in crystalline solids constitute planes (lattice planes) of high electron density. These electrons scatter a monochromatic beam of X-rays generating diffraction maxima via interference in the scattered light. Hence, X-ray diffraction can provide exact information on the spatial relationship between constituent atoms which can be defined most clearly in crystalline substances where the distribution of atoms consists of periodic repetition. This regularity of the atomic arrangement results in the scattered X-rays cancelling each other in most directions and in only certain directions constructive interference will occur (Figure 1). Synthesis and X-ray diffraction of metal phosphate was studied by Xiaobo Pan et al.
Figure 1: Braggs law.
X-ray diffraction: The sample residue from the TGA was analyzed by X-ray diffraction. Sample residues were scraped from the crucible or tungsten disk and put in a silica sample holder and then placed inside the X-ray instrument operating in slow scan mode. The samples were scanned at a speed of 0.25°/min through the angle ranging from 20° to 90°. The X-ray diffraction patterns were collected using Jade V.7 software using condition six.
RESULTS AND DISCUSSION
The solid product synthesized in optimum condition from the reaction of sodium pyrophosphate to copper nitrate was subjected to X-ray diffraction and thermal analysis. During heating up to 600°C, several transformations of the phosphate take place. The analysis is to heat the sample on a tungsten disk and crucible of various concentration 0.1, 1 M concentration of copper phosphate samples were studied (Figure 2).
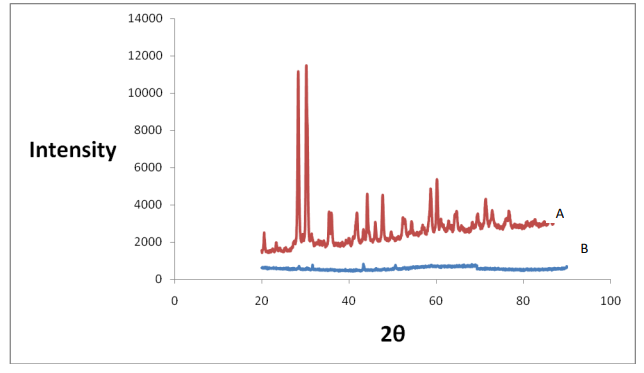
Figure 2: XRD spectra of copper pyrophosphate of 0.1 M concentration after heating in TGA argon A) After heating in TGA argon and B) before heating in TGA argon.
The XRD spectrum of copper phosphate 0.1 M before heating on the TGA is quite ambiguous and after heating on the TGA shows copper pyrophosphate is illustrated in Figure 2 using alumina crucible and argon as the environment in the TGA (Figure 3).
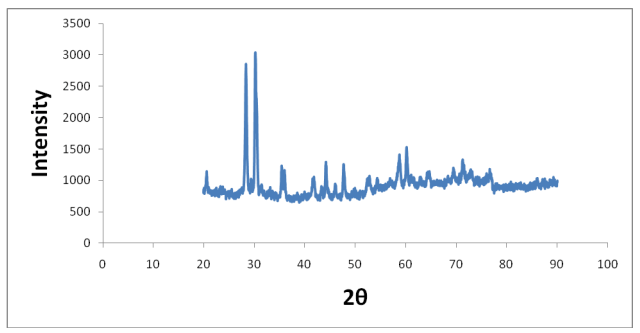
Figure 3: XRD spectrum of copper pyrophosphate 1 M after heating in TGA (air) using crucible.
The copper pyrophosphate of 1 M concentration was used for thermogravimetric analysis and the left-over sample was used for X-ray diffraction study showed the presence of crystalline copper pyrophosphate is shown in the Figure 3 (Figure 4).
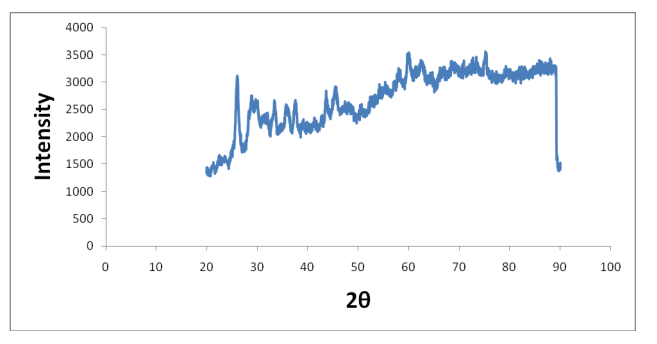
Figure 4: XRD spectra of ambiguous copper phosphate compound 1 M (A) Before heating in TGA (argon); (B) After heating in TGA using tungsten disk a sample of copper phosphate of 1 M concentration was prepared for X-ray diffraction analysis.
In this case the freshly prepared sample showed that the XRD pattern is quite ambiguous as shown in Figure 3. The X-ray diffraction studied after running on the TGA showed that the left-over sample is copper pyrophosphate. Tungsten disk was used as the surface and argon was used as an environment.
The copper pyrophosphate of 1 M concentration was used for thermogravimetric analysis and the left-over sample was used for X-ray diffraction study showed the presence of crystalline copper pyrophosphate is shown in Figure 4 (Figure 5).
Figure 5: XRD of Copper pyrophosphate 0.1 M at (100°C).
The copper pyrophosphate sample dried at 100°C of 0.1 M concentration is made from a totally different phase which is copper orthophosphate is confirmed by X-ray diffraction library is shown in Figure 5.
The XRD result is interesting because all the outcomes match with that of copper pyrophosphate after heating in TGA, whether different environment like argon, air was used or whether different surface like crucible or tungsten disk was used. However, the exact search match for the sample before heating is quite ambiguous.
CONCLUSION
The products synthesized using copper nitrate and sodium pyrophosphate have been studied using a combination of X-ray diffraction and thermal analysis technique. X-ray diffraction shows that at higher temperature of 600°C the anhydrous form of copper pyrophosphate was formed. Copper pyrophosphate compound may lose water and some phase changes occur. The X-ray diffraction pattern of the initial product of two concentrations looks amorphous nature in XRD and shows crystalline behavior after the TGA was done. Sharper and cleaner XRD was obtained from the 0.1 M concentration. Among the two concentrations, some bigger crystals were obtained from 0.1 M concentration. At two different temperatures of 100°C and 600°C the copper phosphate species existed at two different phases which is copper orthophosphate and copper pyrophosphate.
References
- Errandonea D, et al. Cryst Growth Des. 2023;23(4):2782-2794.
[Crossref] [Google Scholar] [PubMed]
- Kiriukhina G, et al. Materials. 2022;15(4):1411.
[Crossref] [Google Scholar] [PubMed]
- Romero-Munniz I, et al. ACS Appl Mater Interfaces. 2021;13(45):54106-54112.
[Crossref] [Google Scholar] [PubMed]
- Williams M, et al. Anal Chem. 1972;44(7):1342-1344.
- Wagner KA, et al. Spectrochim Acta B. 1998;53(11):1507-1516.
- L'vov BV, et al. Spectrochim Acta B. 1995;50(12):1459-1468.
- Krakovska E. Spectrochim Acta B. 1997;52(9-10):1327-1332.
- Krakovska E, et al. Fresenius J Anal Chem. 2000;366:127-131.
[Crossref] [Google Scholar] [PubMed]
- Watanabe M, et al. J Mater Sci. 1990;25:4356-4360.
- Pan X, et al. Dalton Trans. 2017;46(46):16034-16040.
[Crossref] [Google Scholar] [PubMed]
- Ramesh, et al. J Chem Pharm Res. 2015;7(5):793-797.