Original Articles: 2024 Vol: 16 Issue: 2
Copper Coatings in an Alkaline Environment under Organic Polyamines-Complexors Effects
Mamadou Kalan, Diallo*
Department of Chemistry, Hassan II University of Casablanca, Mohammedia. Morocco
- Corresponding Author:
- Diallo
Department of Chemistry,
Hassan II University of Casablanca,
Mohammedia.
Morocco
Received: 16-Jun-2023, Manuscript No. JOCPR-23-102755; Editor assigned: 19-Jun-2023, PreQC No. JOCPR-23-102755 (PQ); Reviewed: 26-Jun-2023, QC No. JOCPR-23-102755; Revised: 27-Dec-2023, Manuscript No. JOCPR-23-102755 (R); Published: 03-Feb-2024, DOI:10.37532/0975-7384.2024.16(01).071.
Citation: Kalan M. 2023. Copper Coatings in an Alkaline Environment under Organic Polyamines-Complexors Effects. J. Chem Pharm. Res., 16:071.
Copyright: © 2024 Kalan M. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Safety and compliance with environmental regulations are major issues for humanity. The compounds used here are not all free consequences for our environment. This is why we must be careful with the use of cyanides which can be very breakneck for our ecosystem. The use of Copper Sulphate Pentahydrate (CuSO4*5H2O) under the effect of organic polyamines-complexors , as well as sodium hydroxide (NaOH) at concentrations varying from (0.25 to 0.375) mol/l, applying low current densities (less than or equal to 0.2 A/dm2), make it possible to obtain very good electrolytic yields of copper coating in a strongly alkaline media without the direct use of cyanide complexing agent in the electrochemical bath on different substrates. The physical characterization carried out by the Fischer Scope X-ray Xulm expedited the determination of the copper electrodeposited thicknesses on the Iron (steel) substrates for the various studied cases and to draw profiles.
Keywords
Copper, Plating, Alkaline media, Polyamines, Fischer Scope X-ray Xulm, Complexors
Introduction
It is well known that coatings of copper can be practically reached in a strongly alkaline medium by means of certain bodies (complexing agents). Bright, ductile, and easily oxidized copper coatings are hold in a very alkaline medium with good thicknesses, using sodium or potassium cyanide and double potassium and sodium tartrate as complexing agents’ cupric ions [1]. However, the use of cyanide based electrolyte baths in an industrial environment is a great peril for terrestrial and aquatic organisms [2]. Moreover, copper coatings can also be beautiful and well acquire in a strongly acid media using various electrolytes reported in the scientific articles/books [3]. Operating with a limited copper plating possibility, because do not allow to copper all substrates. Certain substrates require alkaline copper plating before being conveyed in an acid copper or nickel plating bath, this is the case of substrates made of zinc, zamak, steel, and many others, to avoid the formation of blisters on the deposit or embrittlement by hydrogen gas of the deposit. In addition to the two preceding methods, the deposits of copper can be received from a chemical copper plating baths, using electrolytes and catalysts (reducing agents) very suitable allowing the reduction of copper ions in the solution on the substrate without any supply of an electric power generation during the process. However, this chemical copper plating procedure turns out to be too expensive.
Thus, it would be better to seek one or new solutions to these problems highlighted above. What method should be more favourable to overcome these issues? We note, according to the scientific bibliography and industrial patents, several authors have studied copper plating in different environments using various electrolytes and applying various methods. As described and reported in many journals, organic compound such as polymers containing amine, thiol, phosphorous or oxygen functional groups have several benefits as thoroughly highlighted in Malinauskas A, et al., paper and as well as in uncountable research or industrial patents as presented in handbook of conducting polymers and advances in conductive polymers [4-6]. Organic polyamines, especially have gained more attention in the electrochemical coatings processes and surface functionalization. So that, it shows innumerable advantages in the corrosion protection and surface properties improvement. So, as described and published in many publishing houses, amines and polyamines give attractive interests in the electrochemical plating bath for metal or alloys coatings on diverse substrates. As showed by Katirci R that both Ethylenediamine (ED) and Triethanolamine (TEA) can be used as complexing agent for a favourable electrodeposition of zinc-nickel alloys in alkaline media without any addition of cyanide complexing agent in the process. Recently, in another study conducted by the above author for a statistical approach to optimize zinc-nickel bath contaning (ED) and (TEA) complexing agents, were discovered that (ED) enhanced the particle size of the alloy when the nickel additive was 10 ml/l. Whereas, Ni additive concentration growth from (10 to 20) ml/l in the presence of 45 ml/l of (TEA), (ED) enhanced the smoothness and compactness and in contrast decreased the particle size of the alloy. More recently, Lotfi N mentioned that Zn-Ni alloy coatings has longer history than other zinc composites and many organic compounds such as triethylamine, glycine , polyamines compounds, and glycerol could by applied as complexing and as well as additives for Zn-Ni plating. Moreover, Jiale C, et al., (suggested that heterocyclic compound as aromatic aldehyde and polyamines with epichlorohydrin has higher adsorptivity on the Hg-electrode at potential between (-0.16 ~-1.68) V vs. Hg/HgO in 1 M KOH and are used to produce ZN-11 additive for zinc-nickel plating process (Figure 1) [7-10].
As described in Frischauf R, et al. that for complexing a metal such as Nickel or Copper and so on at high pH, generally alkaline electrolytes must contain complex mixtures of polyamines in order to facilitate the metal salt solubilization and avoid the precipitation of the metal. But, the use of polyamines under electrolysis can form breakdown products at the anode, among which is cyanide. Which hike several issues such as an environmental pollution, negative effect on the bath performance as well as the performance of the deposit decrease. Fortunately, the formation of cyanide upon electrolysis at the anode can be avoided by the utilization of a box membrane apparatus. So that, it seems that organic compounds have large features in the electrochemical plating of metals and their alloys, such as Zn, nickel, copper and Zn-Ni, CuZn, and so on. Hence, in this manuscript we focus on the application of aliphatic polyamines complexors effects for the electrodeposition of copper sulphate in a strong sodium hydroxide solution with free cyanide complexing agents. This work provide a promising new alternative alkaline copper plating methodology, promoting safety and compliance with environmental regulations [11].
MATERIALS AND METHODS
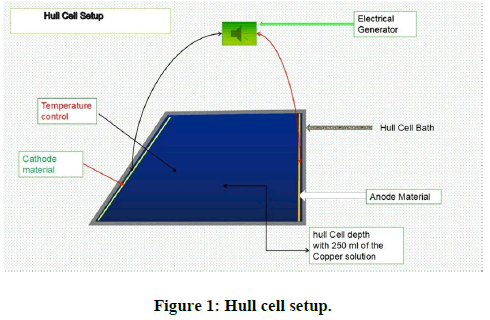
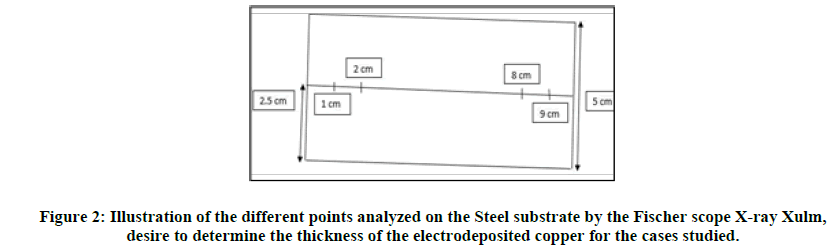
The copper electrodeposition was carried out on different substrates (iron, brass, zinc, zamak, and copper), applying a current density less than or equal to 0.2 A/dm² and a voltage greater than or equal to 2.5 volts. The electrolysis current and voltage generator is a current rectifier with maximum capacity (10 volts and 15 A). The copper electrodeposition time varies from 10 to 15 minutes depending on the studied case and the electrolysis was carried out at room temperature which varies from 18 to 25°C. The electrolyte used was composed of 0.14 mol/l of copper sulphate, (0.25 to 0.375) mol/l of sodium hydroxide, and 100 ml/l of amine mixture (performa 285 base) used as complexing agent to copper ions to prevent their precipitation in the strongly alkaline media with a pH value closed to the region of 14 and 3 ml/l of an additive, added as a brightening agent (performa 285 universal brightener). The materials used as an anode are made of pure copper. The electrochemical cell used is a hull cell with a capacity of 250 ml up to the indication level line, the large distance, the small anode cathode distance, and the depth being 13 cm, 10.5 cm, and 6.8 cm respectively. The cell has a trapezoid shape and is made of plastic material that resists to aggressive products such as acids, bases, and corrosive products. Furthermore, it has good resistance to heated liquids. The different obtained copper coatings are subjected to a visual (shiny, matt, burnt or unburnt), physical characterization (determination of the thickness of the electrodeposited copper in μm at 1 cm, 2 cm, 8 cm, and 9 cm of the hull cell plate) by a Fischer scope X-ray xulm and a mechanical evaluation (ductility test) to see the adhesiveness of the deposited copper on the different substrates (iron, zinc, zamak, brass, and copper) (Figure 2).
RESULTS AND DISCUSSION
The influence of Performa 285 universal brightener on the deposit of alkaline copper
First of all, we tested the effect of the brightener agent on the deposition of alkaline copper with free cyanide, by studying the plating of copper on steel and brass substrates. Indeed, we examined the effects of the brightener during the deposition of copper on steel substrates, while varying the performa 285 universal brightener concentration between three values distributed over three tests: 0 ml/l for the first test (test1), 10 ml/l for the second (test 2) and 20 ml/l for the third test (test 3). The following results were thus obtained for each of these tests (Tables 1-3).
For test 1, the following thicknesses are obtained:
| n=1 | Cu1=1.5 µm |
| n=2 | Cu1=1.3 µm |
| n=3 | Cu1=0.4 µm |
| n=4 | Cu1=0.4 µm |
Table 1: Obtained thickness in test 1.
Average (Cu) 0.88 μm
Standard deviation 0.56 μm
Coefficient. Of variation (%) --------
Extended 1.1 μm
Nb. valuable 4
Minimum value 0.4 μm
Maximum value 1.5μm
Measuring time 10 sec
For test 2, the obtained thicknesses are:
| n=1 | Cu1=1.8 µm |
| n=2 | Cu1=1.7 µm |
| n=3 | Cu1=0.6 µm |
| n=4 | Cu1=0.5 µm |
Table 2: Obtained thickness in test 2.
Average (Cu) 1.12 μm
Standard of deviation 0.70 μm
Coefficient. Of variation (%) --------
Extended 1.3 μm
Nb. valuable 4
Minimum value 0.5 μm
Maximum value 1.8 μm
Measuring time: 10 sec
And for test 3, we had the following values:
| n=1 | Cu1=1.7 µm |
| n=2 | Cu1=1.5 µm |
| n=3 | Cu1=0.5 µm |
| n=4 | Cu1=0.4 µm |
Table 3: Obtained values in test 3.
Average (Cu) 1.02 μm
Standard of deviation 0.65 μm
Coefficient. Of variation (%) --------
Extended 1.2 μm
Nb. valuable 4
Minimum Value 0.4 μm
Maximum Value 1.7 μm
Measuring time 10 sec
NB: The thicknesses of the copper deposit are obtained by direct measurement, using an impedance device (Fischer scope x-ray xulm). And the deposition time is taken as equal to 15 minutes.
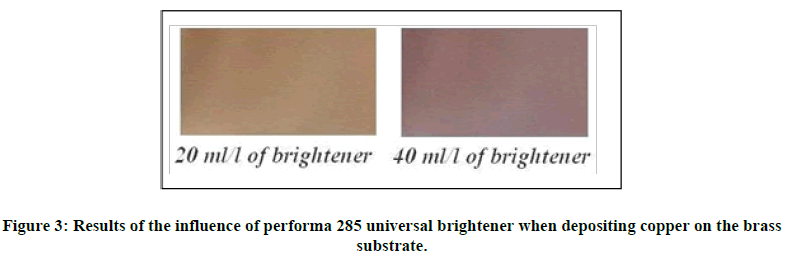
We note that the average thickness obtained for (test 2) is greater than that obtained for (test 1) and (test 3). For all the tests (1, 2, and 3) carried out, the obtained deposit is very bright, but that obtained for (test 3) is brighter compared to (test 2) which is also in turn brighter than the obtained deposit at (test 1). The addition of 10 ml/l of performa 285 universal brightener made it possible to accelerate the deposition of copper by 21% (of thickness) compared to the case presented with an absence of this brightener agent. For high values of the brightener agent exceeding the value (10 ml/l), a reduction of the copper thickness is noted, so in the case of 20 ml/l of performa 285 universal brightener is added, a reduction of 8.93% in the thickness of copper is reached. Thus, it can be said that, to obtain better yields of alkaline copper deposition with free-cyanide on iron substrates (steels), the value (10 ml/l) of performa 285 universal brightener will be very suitable. In a second step, we examined the influence of the same brightener during the deposition of copper on brass substrates, by varying the concentration of the brightener. In this case only two experiments were done: (20 ml/l) and (40 ml/l) of performa 285 universal brightener corresponding to experimental test 4 and 5 respectively. From the two previous experiments, the obtained results are shown in the two images below (Figure 3).
It is noted that the change in concentration of brightener has a great influence during the electroplating of copper on brass substrates. This influence is characterized by a change in color of the deposited copper as a function of the value of brightener concentration. In our case, we can see in the two images above, the color change from the copper deposit which is pale orange for a concentration equal to (20 ml/l) to becomes chocolate purple once we change the concentration to reach the value (40 ml/l) of brightener. For desired characteristics, it is therefore sufficient to vary the concentration of the brightener towards an objective value.
Influence of Sodium Hydroxide (NaOH) on the deposit of alkaline copper
In this part, the copper deposit was carried out with two baths, one made at a concentration equal to 0.25 mol/l of (NaOH) and the other at 0.375 mol/l, both baths gave good results. As for the study of the influence of sodium hydroxide on the deposition of alkaline copper with free cyanide, two tests were made, in the first test (test 6), 5 ml of a concentrated sodium hydroxide solution (2.5 mol/l) were added to 250 ml of the alkaline copper solution with free cyanide (increased by 0.05 mol/l of caustic soda). And in the second test (test 7) we added as in the previous test (test 6)10 ml of the sodium hydroxide solution of concentration (2.5 mol/l) in 250 ml of the solution of alkaline copper(increased by 0.1 mol/l caustic soda) respectively. The results obtained concerning the influence of sodium hydroxideon the deposition of alkaline copper during these two tests (6 and 7) are given here:
For the experiment carried out with the addition of (5 ml of the NaOH solution at (2.5 mol/l)) or test 6, the following thicknesses are noted after 10 minutes of electroplating of alkaline copper free cyanide (Table 4).
| n=1 | Cu1=1.2 µm |
| n=2 | Cu1=1.2 µm |
| n=3 | Cu1=0.5 µm |
| n=4 | Cu1=0.4 µm |
Table 4: Obtained thickness of electroplating of alkaline copper free cyanide after 10 minutes in test 6.
Average (Cu1) 0.82 μm
Standard of deviation 0.44 μm
Coefficient. Of variation (%) --------
Extended 0.9 μm
Nb. valuable 4
Minimum value 0.4 μm
Maximum 1.2 μm
Measuring time: 10 sec
For the experiment carried out with the addition of (10 ml of Na OH solution at (2.5 mol/l)) or test 7, the following thicknesses are obtained after 10 minutes of electroplating (Table 5).
| n=1 | Cu1=1.3 µm |
| n=2 | Cu1=1.2 µm |
| n=3 | Cu1=0.7 µm |
| n=4 | Cu1=0.4 µm |
Table 5: Obtained thickness of electroplating after 10 minutes of test 7.
Average (Cu1) 0.92 μm
Standard of deviation 0.43 μm
Coefficient. Of variation (%) --------
Extended 0.9 μm
Nb. valuable 4
Minimum value 0.4 μm
Maximum value 1.3 μm
Measuring time 10 sec
Thus, we notice, a difference in the thickness of alkaline copper deposit with free-cyanide for the two tests (6 and 7). According to the average obtained thickness, the calculated thickness difference is 0.1 micrometer, that is about (10%) of the thickness. Moreover, there is an increase in the concentration of caustic soda in the same direction as that of the increase in the thickness of copper plating. After a careful examination of the obtained deposit appearance for the two experiments (test 6 and 7), we observe a poor quality of the copper deposit (blackish deposit) at a high current density for high caustic soda concentrations (See image below (c)). However, in experiments carried out at the same potential but with lower concentrations of caustic soda than the concentrations of the test (6 and 7), there is a deposit with a good quality (deposit appearance is of shiny copper) without burning at high current density. Consequently, working at caustic soda concentrations ranging from (0.25 to 0. 375) mol/l would be preferable, in order to maintain a good copper plating yield (appearance and thickness), according to the average thicknesses obtained in the various tests carried out previously (Figure 4).
Deposit on different substrates
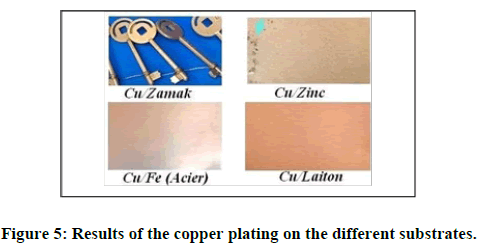
The electrodeposition of copper from prepared baths was carried out on various substrates such as brass, nickel, zinc, iron (steel), aluminum, and zamak. However, only brass, nickel, steel, and zamak substrates have given us good results. The images below illustrate the obtained copper deposit on certain supports (materials) (Figure 5).
We note for the case of the support in (zinc), the copper does not adhere well, especially at high current density even the indication light blue arrow (image (Cu/Zinc)); on the other hand, it adheres well to the zamak substrate and very well for the other substrates (copper, brass, iron, and nickel).
Deposition of metals on the deposit of alkaline copper
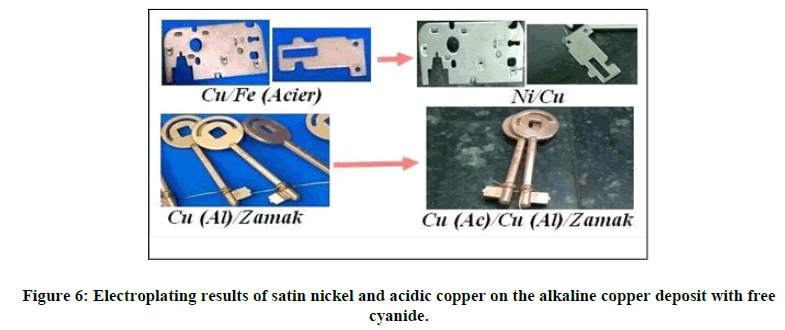
In this part, a good deposit of satin nickel was obtained on alkaline copper substrates with free cyanide. Likewise, acid copper plating was carried out on alkaline copper substrates with free cyanide (from our prepared baths). The image below allow us to demonstrate the obtained results (Figure 6).
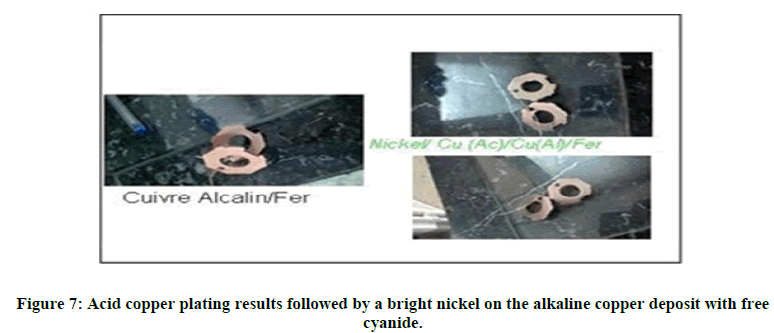
We notice in both cases, the result obtained is of better quality, so we can use this type of copper plating as a pre- treatment for brass processes, acid copper plating, shiny and satin nickel, and As well as other processes requesting an undercoat of alkaline copper. In addition, bright nickel has been deposited on the acid copper coating which covers the deposition of alkaline copper without direct use of cyanide complexors on steel substrates, as illustrated in the below Figure 7.
These results show that this alkaline copper free cyanide process can be applied to obtain a copper undercoat for different substrates, in order to facilitate the subsequent treatments which are necessary for numerous substrates.
CONCLUSION
In order to provide a solution related to a safety problem and compliance with environmental regulations, we considered carrying out this work, to avoid the use of electrolytic baths of alkaline copper plating based on Copper Cyanide (CuCN), Sodium Cyanide (NaCN) or Potassium Cyanide (KCN). The use of copper sulphate pentahydrate (CuSO4*5H2O) under the effect of organic polyamines complexors has made it possible to obtain a very good deposition of copper on various substrates such as (iron, brass, and nickel) while the substrates made of zamak and zinc have yields lower. It is noted that the increase in the concentration of Sodium Hydroxide (NaOH) promotes an increase in the current density and therefore the thickness of the electrodeposited copper. However, a high current density leads to the formation of a blackish deposit on the short anode cathode distance side of the hull cell (the part very close to the anode (high current density on this side)). In addition, it is noted that different materials can be electrodeposited on the obtained alkaline copper deposit under organic polyamines effects, as shown in the images (Ni/Cu) and Cu(Ac)/Cu(Al). The use of performa 285 universal brighteners depends on the substrate and its condition, as we have seen for steel substrates the concentration was (3 to 20 ml/l) while on brass substrates, the concentration can go up to (40 ml/l) with satisfactory results. In short, working at low concentrations of caustic soda, medium concentrations of brightening agent and low voltage (less than or equal to 2.5 volts) as well as at low current density (less than or equal to 0.2 A/dm²) will make it possible to yield a very good deposit of copper in an alkaline medium under the effect of using organic polyamines without the direct use of cyanide complexors with good electroplating yields.
CONFLICT OF INTEREST
The author declares that this experimental paper was led in the absence of any marketable or economic relationships that could be interpreted as a potential conflict of interest.
References
- Yves, et al. Techniques Ingenieur. 1982;1:605.
- Renard C, et al. John Libbey Eurotext Publications. Newyork, USA. 2005:353-359.
- Abd El Rehim SS, et al. Appl Surf Sci. 2000;165(4):249-254.
- Malinauskas A, et al. Nanotechnology. 2005;16(10):81.
[Crossref] [Google Scholar] [PubMed]
- Skotheim TA, et al. CRC press. Florida, United States. 1997.
- Kumar D, et al. Eur Polym J. 1998;34(8):1053-1060.
- Katirci R. Surf Eng. 2015;31(1):11-16.
- Katirci R. Surf Rev Lett. 2015;22(01):1550015.
- Lotfi N, et al. Prot Met Phys Chem. 2018;54:1102-1140.
- Jiale C, et al. J Electrochem. 1995;1(3):332.
- Frischauf, et al. AESF publications. Florida, United States. 2002.