Short communication: 2025 Vol: 17 Issue: 1
Considerations for Equipment Choice in Cell Therapy Workflows
Maetja Verbarendse, Richard Snyder, Uma Lakshmipathy*
Department of Science and Technology, Pharma Services, Thermo Fisher Scientific, California, United States
*Corresponding Author:
- Uma Lakshmipathy
Department of Science and Technology, Pharma Services, Thermo Fisher Scientific, California, United States
Received: 26-Aug-2023, Manuscript No. JOCPR-23-111296; Editor assigned: 29-Aug-2023, PreQC No. JOCPR-23-111296 (PQ); Reviewed: 12-Sept-2023, QC No. JOCPR-23-111296; Revised: 02-Jan-2025, Manuscript No. JOCPR-23-111296 (R); Published: 09-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).237.
Citation:Lakshmipathy U, et al. 2024. Considerations for Equipment Choice in Cell Therapy Workflows. J Chem
Pharm Res., 17:237.
Copyright: © 2025 Lakshmipathy U, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Cell therapies are a rapidly growing market with a complex bio-manufacturing process. This necessitates the need for robust processes that can be implemented during process establishment to enable scalable and reproducible GMP manufacturing. The development of new platforms designed for specific cell therapy applications can add immense value, improving efficiency, purity, and potency, however, integration of new instruments in GMP processes requires a risk-based approach. Adopting these platforms into new workflows is critical to match the speed of therapeutic product innovations and manufacturing requirements. In this commentary, we summarize our recent publication that highlighted the critical considerations for equipment implementation into a translatable cell therapy workflow, evaluating features based on process suitability and compliance to regulatory requirements.
Keywords
Bio-manufacturing, Cell therapy, Equipment evaluation, Good Manufacturing Practices (GMP), Translational services
Introduction
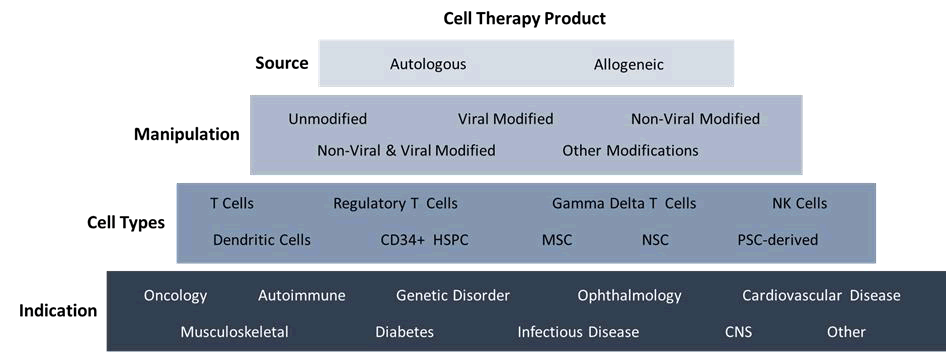
Cell and gene therapy products are a rapidly emerging market that require an accelerated pace of discovery and translation into cGMP manufacturing processes and analytics. Due to the diverse nature of these complex workflows used to address different clinical indications (Figure 1), there is a need for a wide choice of equipment options with different features [1]. To meet this demand, the market is flooded with new or improved instruments to outfit cell therapy manufacturing suites. Although this can provide beneficial options for the end-user; it also poses a dilemma [2]. User’s must choose equipment that fits their required specifications, while balancing the complexities that may be arise from additional instrument maintenance, consumables, and training [3].
The choice of general lab equipment such as refrigerators, freezers, and biosafety cabinets are standard depending on their features and compatibility with the facility layout [4]. Similarly, analytical equipment choice is driven by the assay required for in-process testing and QC release [5]. To meet cleanroom and process requirements, a more thorough assessment is needed for specialized equipment that directly contact the cellular product [6].
Figure 1: Diversity of cell therapy workflows and varying manufacturing requirements.
A newly launched instrument is considered appropriate when its specifications satisfy regulatory compliance constraints, meets user requirements and product-specific process performance [7]. Further, the ease in integrating a new or different instrument in a workflow depends on the maturity of the project, with substitutions in later clinical stages being more difficult. During early stages, processes and scale are yet to be defined and there is greater flexibility to introduce new instruments and methods. After significant manufacturing development, alternate equipment and methods may only be considered if current systems are inadequate [8]. At advanced stages where processes are locked for commercial manufacturing, a change from one instrument to another is not trivial.
Description
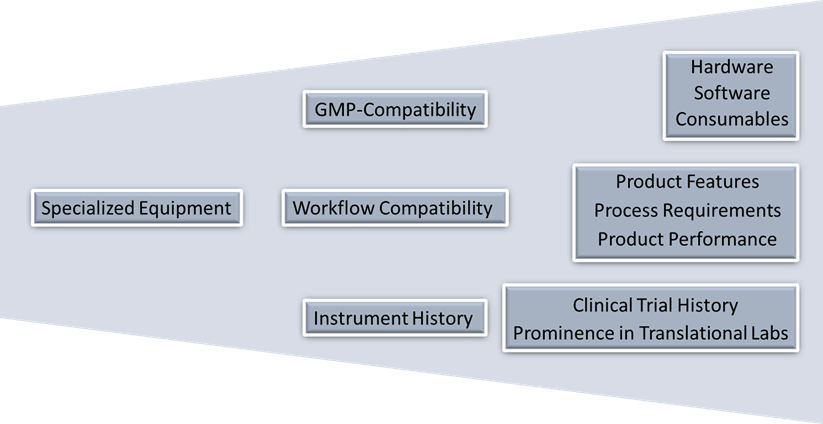
The notion of best-in-class equipment implies that the instrument can support a GMP-compliant manufacturing process while fulfilling the user requirements to generate high quality cell therapy products in a consistent manner. These requirements can be broadly classified as
• GMP-compatibility of hardware, software, and consumables;
• Workflow compatibility that can deliver the necessary scale and purity of cells as defined by the process, and
• Instrument history and prominence of the equipment (Figure 2).
Figure 2: General requirements for specialized cell therapy equipment that directly contact the cellular product.
Newly launched equipment lacks performance and clinical trial history which can be a barrier when comparable equipment choices that have already been successfully used in clinical trials and are familiar to regulatory agencies. Process performance of new equipment can be based on vendor data or an early research publication, but its performance in the production or analysis of a specific cell therapy product is paramount. The prominence of newer equipment in multiple cell therapy development laboratories and proven use preferably in a cGMP manufacturing setting can accelerate the implementation and integration of new equipment.
A check list of these factors along with a methodology to score and weigh each attribute is a valuable decision-making tool to rationally assess the suitability of equipment in GMP cell therapy manufacturing and analysis. In our recent paper published in CytoTherapy1, a 0 to 5 point scale was used in an un-weighted manner. However, this grading system can be customized based on the level of rigor needed by weighting categories based on criticality of each feature to a user’s process.
Conclusion
Cell and gene therapy products represent a rapidly emerging market that necessitates a swift transition from discovery to cGMP manufacturing processes and analytics. The complexity and diversity of workflows addressing various clinical indications require a wide range of equipment options with distinct features. Consequently, the market is inundated with new or improved instruments designed to outfit cell therapy manufacturing suites. While this proliferation offers beneficial choices for end-users, it also presents a significant challenge: selecting equipment that meets specific requirements while balancing complexities related to maintenance, consumables, and training.
Standard laboratory equipment choices, such as refrigerators, freezers, and biosafety cabinets, are generally straightforward, based on their features and compatibility with the facility layout. In contrast, selecting analytical equipment is driven by the specific assays required for in-process testing and QC release. Specialized equipment that directly contacts cellular products demands a thorough assessment to meet cleanroom and process requirements. A newly launched instrument must satisfy regulatory compliance, meet user requirements, and deliver product-specific process performance. The maturity of the project significantly influences the ease of integrating new instruments; early stages allow more flexibility, while later stages require more rigorous justification for changes.
The concept of best-in-class equipment implies that an instrument can support GMP-compliant manufacturing processes and meet user requirements to consistently produce high-quality cell therapy products. These requirements encompass GMP-compatibility of hardware, software, and consumables; workflow compatibility for delivering the necessary cell scale and purity; and the instrument's history and prominence in the field. Newly launched equipment often lacks extensive performance and clinical trial history, which can be a barrier when comparable, proven equipment is available. Performance data from vendors or early research publications is crucial, but real-world performance in a cGMP setting is paramount.
A comprehensive checklist of factors, alongside a methodology to score and weigh each attribute, serves as a valuable decision-making tool for assessing equipment suitability in GMP cell therapy manufacturing and analysis. A customizable grading system, based on the criticality of each feature, can provide the necessary rigor for making informed equipment choices. This approach ensures that the chosen instruments not only comply with regulatory standards but also enhance the efficiency and quality of cell therapy manufacturing processes.
References
- Verbarendse M, et al. Cytotherapy. 2023;25(10):1107-1112.
[Crossref] [Google Scholar] [PubMed]
- Harrison RP, et al. Biotechnol Adv. 2018;36(2):345-357.
[Crossref] [Google Scholar] [PubMed]
- Kirouac DC, et al. Cell Stem Cell. 2008;3(4):369-381.
[Crossref] [Google Scholar] [PubMed]
- Martin I, et al. Trends Biotechnol. 2005;23(12):564-572.
- Rafiq QA, et al. Curr Opin Chem Eng. 2013;2(1):8-16.
- McKee C, et al. Colloids Surf B Biointerfaces. 2017;159:62-77.
[Crossref] [Google Scholar] [PubMed]
- Kaiser AD, et al. Cancer Gene Ther. 2015;22(2):72-78.
[Crossref] [Google Scholar] [PubMed]
- Heathman TRJ, et al. Regen Med. 2015;10(1):49-64.
[Crossref] [Google Scholar] [PubMed]