Original Articles: 2022 Vol: 14 Issue: 3
Cetyl tri-methylammonium Bromide Encapsulated Lawsone (Extracted from Lawsonia inermis) Modified Platinum Electrode as a Voltammetric and Electrochemical Impedance Spectroscopy Sensor for Cholesterol
Bhattacharjya R1*, Bordoloi P2, Das DK2
1 Department of Chemistry, Pandu College, Guwahati, Assam, India
2 Department of Chemistry, Gauhati University, Guwahati, Assam, India
- Corresponding Author:
- Bhattacharjya R
Department of Chemistry, - Pandu College,
Guwahati,
Assam,
India
Received: 07-Mar-2022, Manuscript No. JOCPR-22-53638; Editor assigned: 09-Mar-2022, PreQC No. JOCPR- 22-53638 (PQ); Reviewed: 23-Mar-2022, QC No. JOCPR-22-53638; Revised: 28-Mar-2022, Manuscript No. JOCPR-22-53638 (R); Published: 07-Apr-2022, DOI:10.37532/ 0975-7384-22.14.020.
Abstract
Lawsone (2-hydroxy-1,4-naphthoquinone) is the main active chemical constituent of Henna (Lawsonia inermis). Lawsone encapsulated in cationic surfactant cetyltrimethylammonium bromide modified pre-cleaned platinum electrode, coated with AgNO3, showed a decrease in cathodic and anodic currents in cyclic voltammetry and the peak current in square wave voltammetry. Electrochemical Impedance Spectroscopy of the modified electrode showed a linear increase in charge transfer resistance with cholesterol. The fluorescence intensity of Lawsone in ethanol-water was found to quench in presence of AgNO3 which then gradually increased on addition of cholesterol. The electrochemical behaviour of the modified electrode and the fluorescence behaviour of Lawsone in presence of AgNO3 towards cholesterol were found to be similar in artificial cerebrospinal fluid, confirming that NaCl, NaHCO3, KCl, CaCl2, glucose and uric acid do not interfere with the detection of cholesterol. The limit of detection of cholesterol in 0.1 M KCl is 4.0×10-7 M and 7.76 × 10-8 M in synthetic cerebrospinal fluid.
Keywords
Cholesterol; Lawsone; Voltammetry; Impedance; Fluorescence
Introduction
Molecular recognition has become important in the fields of environmental pollution, medical diagnosis, etc. A change in a property of the probe molecule observable by various instrumental techniques upon interaction with the target molecule is the basic principle for molecular recognition. H-bonding is well exploited means of interaction between probe and the target [1-4]. Study of the change in redox potential and redox currents of the probe on its interaction with the target is well studied for qualitative and quantitative detection of the target [5]. The basic principle of electrochemical biosensor is that chemical reactions between immobilized biomolecule and target analyte produce or consumes ions or electrons, which affects measurable electrical properties of the solution, such as electric current or potential [6]. Designing of modified electrodes for the detection of molecules and ions is a challenging problem which has good analytical applications in environmental chemistry, medicinal chemistry, etc.
Quinones are widespread in living organisms, performing a variety of biochemical and physiological functions. The biochemical functions of quinones are connected to a great extent with their ability to undergo reversible redox conversion and therefore the investigation of their electrochemical properties has aroused significant interest [7]. 2- Hydroxy-1,4-naphthoquinone (HNQ; Lawsone) is the principal natural dye contained at 1.0%-1.4% (high proportion) in the leaves of Henna (Lawsonia inermis) [8,9]. The yellow pigment lawsone is contained in the leaves of the tropical bush Lawsonia inermis (henna plant). Henna has been used for more than 4000 years not only as a hair dye, but also as a body paint and tattoo dye. When henna is applied in a form of paste onto hair, skin or nail, it imparts a reddish-brown coloration lasting for up to twelve weeks. The green leaves paste has been used to prevent various infections such as ulcers, constipation, anemia, small pox and inflammation from ancient time.
Lawsone (2-hydroxy-1,4-naphthoquinone; red-orange dye; natural orange 6; slightly water soluble; simple molecule with non-ionic groups) is the main active chemical constituent of henna leaves that has an affinity towards protein binding, leading to a strong stain at ambient conditions and also used for dyeing in textile industries. Hydroxy naphthoquinone and its derivatives have strong coordination ability towards metal ions and act as redox active ligands10. Kumar and hisco-workers used lawsone in the synthesis of azo dye and prepared a colorimetric sensor for the detection of copper (II) and iron (III) ions by using fluorescence spectroscopy [10,11].
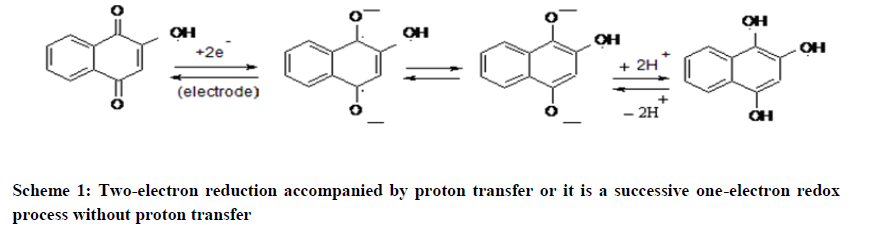
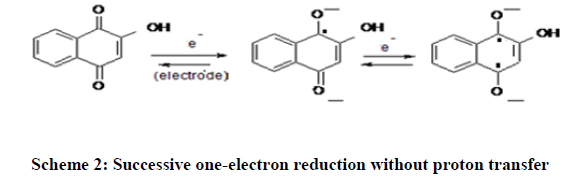
It is reported that Lawsone shows well-defined cyclic voltammogram due to the reversible conversion of the two ketone groups into hydroxyls [12,13]. The electrochemical activity (redox activity) of Lawsone is due to rapid reversible redox equilibrium between oxidized quinone form and reduced hydroquinone form [14]. It is a twoelectron redox process as shown in the Schemes 1 and 2 below:
Naphthoquinones have many physiological roles, because some members of this group as ubiquinone, plastoquinone and K vitamins are key functional constituents of biochemical systems.
Cholesterol has a number of important functions in the human body. Cholesterol and its derivatives are the structural components of biological membranes as well as nerve and brain cells [15]. Cholesterol is synthesized in the liver and also supplied from dietary intake. Besides it takes part in the production of steroid hormones, vitamin D, bile acids and other biologically active molecules [16]. The disorder of cholesterol biosynthesis increases the risk of development of cardiovascular diseases and worsens the function of the immune system. High cholesterol level in blood is a marker of CVDs. Therefore, cholesterol determination techniques are necessary for clinical practice.
Electrochemical techniques are widely spread in test systems and sensor construction. There are very few reported electrochemical methods for the detection of cholesterol.
There have been reports that Ag+ ions can interact with Lawsone [17] as well as with cholesterol [18]. Ag+ can form a strongly bound complex with Lawsone. Moreover, Zaheer, et al. reported that Lawsone adsorption to a surface is much better in the presence of Ag+ ions and CTAB [17]. Again, the presence of Ag+ causes a decrease in the blood cholesterol levels [18].
In this work, we report that platinum working electrode when modified with lawsone (extracted from Lawsoniainermis) encapsulated incetyl trimethyl ammonium bromide and coated with AgNO3 acts as voltammetric and electrochemical impedance spectroscopic sensor for cholesterol. Aqueous solution of Lawsoniainermis and AgNO3 also shows significant emission wavelength shift on interaction with cholesterol.
Materials and Methods
Cetyl Trimethyl Ammonium Bromide (CTAB), Tetra Butyl Ammonium Perchlorate (TBAP), Cholesterol, Glucose, Urea, AgNO3, KCl, NaCl, CaCl2 and NaHCO3 are purchased from Merck and employed as received.
The electrochemical measurements are done using CHI 660D CH electrochemical analyser (USA) consisting of a three-electrode system: Bare or modified Pt electrode as the working electrode, Ag/AgCl (3 M NaCl) as the auxiliary electrode, Pt wire as the reference electrode and Tetra Butyl Ammonium Perchlorate (TBAP) or KCl as the supporting electrolyte. The fluorescence spectra are recorded in Hitachi F-7000 Fluorescence Spectrophotometer using quartz cuvette (1 cm path length).
Extraction of Lawsone (L) from Lawsonia inermis (Henna)
The fresh leaves of Lawsonia inermis (henna plant) were dried at room temperature for 7 days and then crushed into powder form. Lawsonia inermis powder was mixed with ethanol and left for a week. Then the mixture was filtered and Lawsone was extracted by means of the rotary evaporator. The residue left is used for further experiments.
Preparation of L@CTAB/Pt electrode: Lincorporated CTAB (L@CTAB) film on Pt electrode is prepared by dissolving 0.1 g CTAB, 0.1 g TBAP as supporting electrolyte and 0.5 g extracted L in 10 mL absolute alcohol. Then the mixture was stirred for 1 hour and the suspension was filtered. 40 μL of the solution was then placed on the tip of a pre-cleaned Pt electrode surface using Hamilton micro syringe. The solvent was allowed to evaporate resulting in the formation of Lencapsulated CTAB film on the electrode surface. The fabricated electrode will be designated as Pt/L@CTAB working electrode. Cyclic voltammograms were recorded by immersing the tip of this modified electrode in 0.1 M KCl aqueous solution.
Preparation of cholesterol solution: 0.1 M Cholesterol solution was prepared by dissolving 0.387 g of cholesterol in 10 mL absolute alcohol.
Preparation ofPt/L@CTAB/AgNO3 electrode: The tip of Pt/L@CTAB electrode was dipped in saturated aq. AgNO3 solution for 1 hour and allowed to dry naturally. The resulting electrode designated as Pt/L@CTAB/AgNO3 was used as cholesterol sensor.
Preparation of synthetic Cerebrospinal fluid: 0.1 mg urea, 2.8 mg KCl, 3.2 mg CaCl2, 8 mg glucose, 16 mg NaHCO3 and 84 mg NaCl are mixed into 10 mL aqueous solution to prepare the synthetic cerebrospinal fluid [19].
Results and Discussion
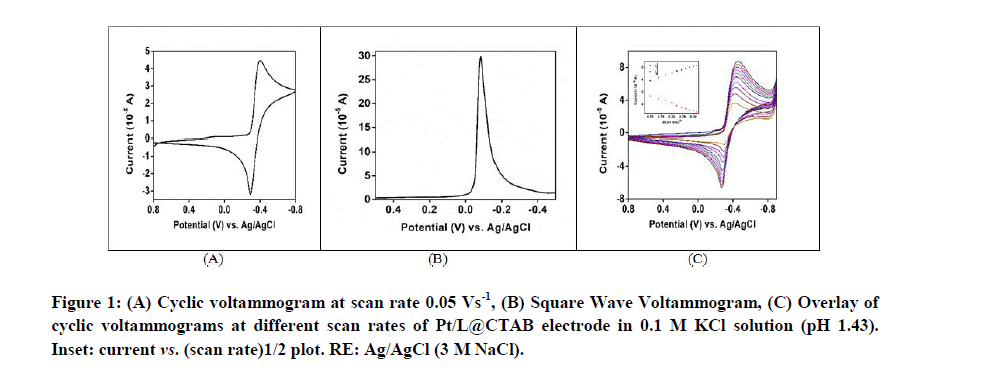
Figure 1A shows the cyclic voltammograms of Pt/L@CTAB electrode in water in presence of 0.1 M KCl as supporting electrolyte at scan rate 0.05 Vs-1 versus Ag/AgCl (3 M NaCl) as reference electrode. A quasi-reversible redox couple with redox potential value E1/2=-0.348 V was observed with ΔE=0.110 V. Figure 1B shows the square wave voltammograms of Pt/L@CTAB modified electrode in water in presence of 0.1 M KCl as supporting electrolyte having similar redox potential value at -0.314 V. Figure 1C shows that with the increasing scan rates both the cathodic and anodic currents of the reversible redox couple increase. The cathodic and anodic currents are found to increase linearly with scan rates (Figure 1C, inset) which is a characteristic of surface adsorption and supports the formation of film on electrode surface (Figure 1).
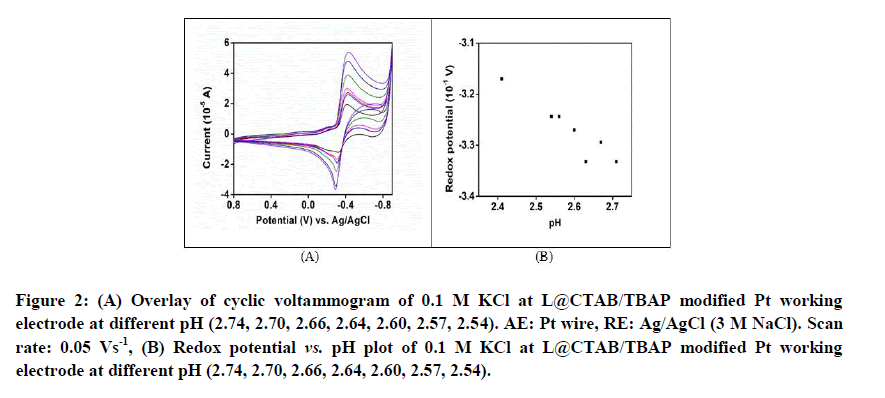
Cyclic voltammetric experiments are done at different pH (2.74, 2.70, 2.66, 2.64, 2.60, 2.57, 2.54) at L@CTAB/TBAP modified Pt working electrode. The cathodic and anodic peak currents are found to increase with decreasing pH which indicates that the redox process becomes faster on decreasing pH (Figures 2A and 2B).
Figure 2: (A) Overlay of cyclic voltammogram of 0.1 M KCl at L@CTAB/TBAP modified Pt working electrode at different pH (2.74, 2.70, 2.66, 2.64, 2.60, 2.57, 2.54). AE: Pt wire, RE: Ag/AgCl (3 M NaCl). Scan rate: 0.05 Vs-1, (B) Redox potential vs. pH plot of 0.1 M KCl at L@CTAB/TBAP modified Pt working electrode at different pH (2.74, 2.70, 2.66, 2.64, 2.60, 2.57, 2.54).
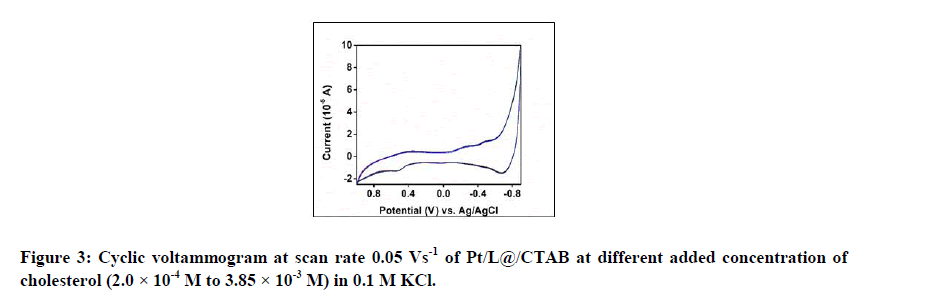
Figure 3 shows the cyclic voltammogram at scan rate 0.05 Vs-1 of Pt/L@/CTAB at different added concentration of cholesterol (2.0 × 10-4 M to 3.85 × 10-3 M) in 0.1 M KCl. No characteristic change is observed.
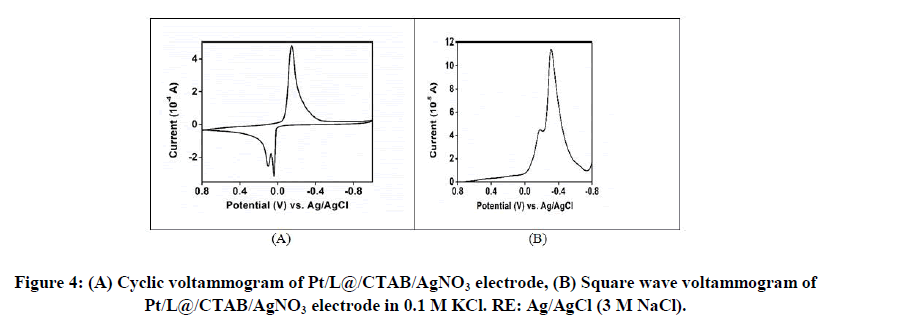
Figures 4A and 4B shows the cyclic voltammogram and square wave voltammogram of Pt/L@CTAB/AgNO3 in aqueous 0.1 M KCl. In cyclic voltammogram one sharp reduction peak was observed at -0.147 V versus Ag/AgCl while two sharp closely spaced peaks were observed for the oxidation process. In square wave voltammogram experiment only one sharp peak was observed at -0.084 V versus Ag/AgCl (3 M NaCl).
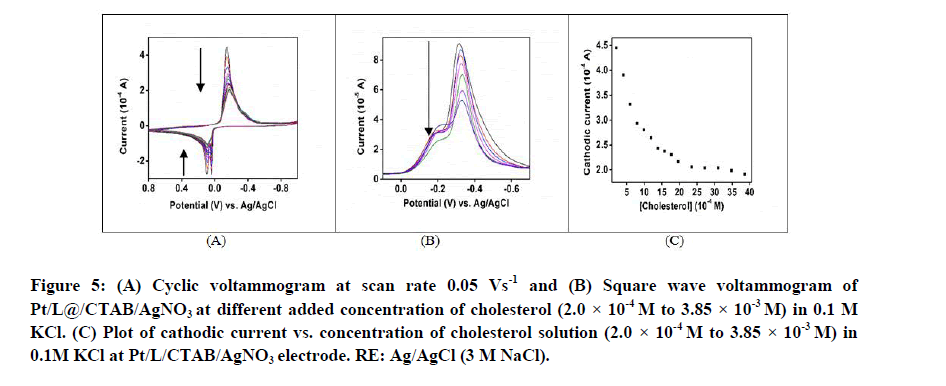
Figure 5A shows the cyclic voltammogram of Pt/L@CTAB/AgNO3 electrode in 0.1 M KCl at different added concentration of cholesterol. Both the oxidation and reduction currents were found to decrease with the concentration of cholesterol. Similarly in square wave voltammogram also the peak current was found to decrease with cholesterol concentration (Figure 5B). The plot of cathodic current versus cholesterol concentration (Figure 5C) shows a linear decrease in current for cholesterol concentration 2.0 × 10-4 M to 3.85 × 10-3 M. The Limit of Detection (LOD) was calculated as per reported method [20] and found to be 4.0 × 10-7 M.
Figure 5: (A) Cyclic voltammogram at scan rate 0.05 Vs-1 and (B) Square wave voltammogram of Pt/L@/CTAB/AgNO3 at different added concentration of cholesterol (2.0 × 10-4 M to 3.85 × 10-3 M) in 0.1 M KCl. (C) Plot of cathodic current vs. concentration of cholesterol solution (2.0 × 10-4 M to 3.85 × 10-3 M) in 0.1M KCl at Pt/L/CTAB/AgNO3 electrode. RE: Ag/AgCl (3 M NaCl).
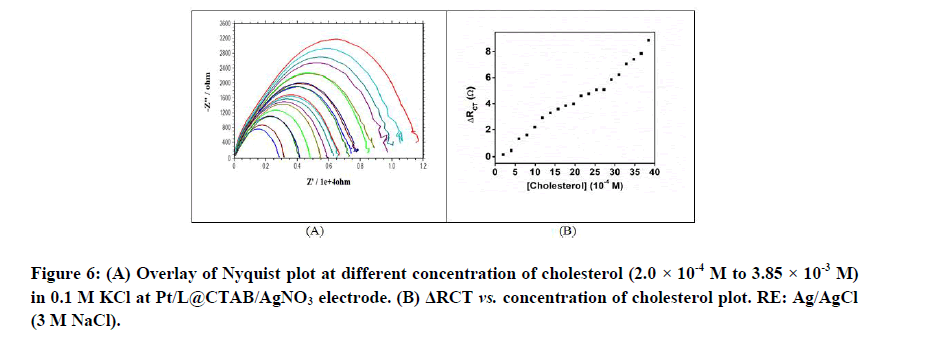
The charge transfer resistance of the 0.1 M KCl solution increased with the gradual addition of cholesterol (2.0 × 10-4 M to 3.85 × 10-3 M), implying a decrease in the current detected in the solution at Pt/L@/CTAB/AgNO3 electrode (Figures 6A and 6B).
A probable explanation for the decrease in reduction and oxidation current without any change in peak positions with cholesterol concentration may be due to the formation of an electro-inactive adduct between silver salt of lawsone and cholesterol through hydrogen bonding. An alternative explanation may be that, when cholesterol is added gradually to the solution, the conductivity of the electrode surface decreases. This may be caused due to the deposition of material onto the electrode surface, thereby increasing the resistance and decreasing the current.
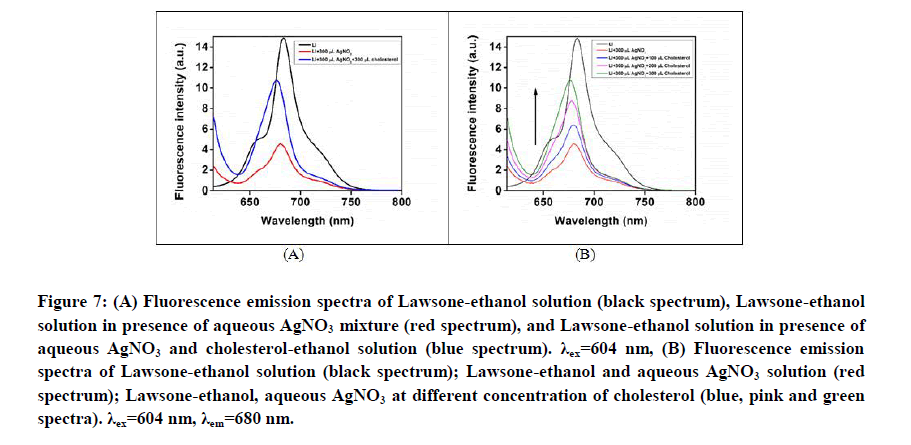
The interaction between cholesterol and Lawsone/AgNO3 solution was further verified by fluorescence studies. The Lawsone in ethanol solution shows fluorescence emission maxima at 680 nm when excited with 604 nm photons. Upon addition of aqueous AgNO3 solution, the fluorescence of Lawsonequenches. Gradual addition of cholesterol solution to this mixture shows an enhancement in the fluorescence intensity accompanied by a small blue shift to 676 nm (Figures 7A and 7B).
Figure 7: (A) Fluorescence emission spectra of Lawsone-ethanol solution (black spectrum), Lawsone-ethanol solution in presence of aqueous AgNO3 mixture (red spectrum), and Lawsone-ethanol solution in presence of aqueous AgNO3 and cholesterol-ethanol solution (blue spectrum). λex=604 nm, (B) Fluorescence emission spectra of Lawsone-ethanol solution (black spectrum); Lawsone-ethanol and aqueous AgNO3 solution (red spectrum); Lawsone-ethanol, aqueous AgNO3 at different concentration of cholesterol (blue, pink and green spectra). λex=604 nm, λem=680 nm.
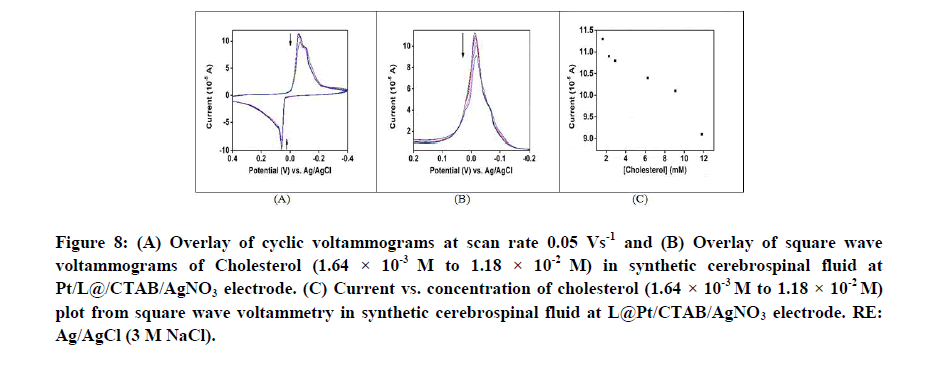
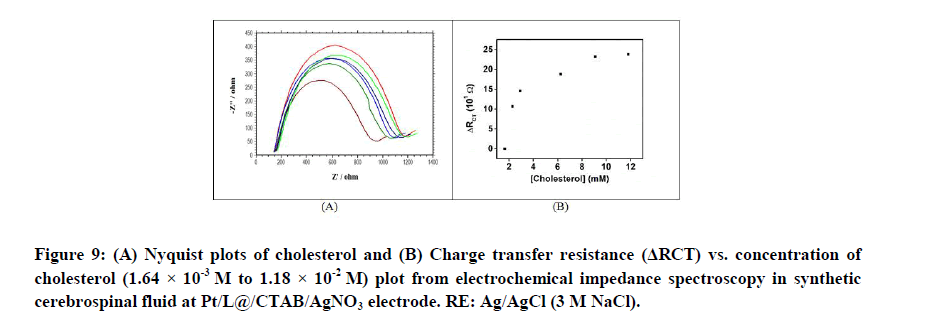
Voltammetry and impedance spectroscopy in synthetic cerebrospinal fluid: Study of interference from other ions and molecules: Cyclic voltammogram, square wave voltammogram and Nyquist plots are measured for the modified electrodes versus cholesterol concentration in presence of synthetic cerebrospinal fluid. The recorded cyclic voltammogram, square wave voltammogram and Nyquist plots were similar to those when recorded with cholesterol alone (Figures 8A-8C) (9A and 9B). This shows that NaCl, NaHCO3, KCl, CaCl2, glucose and urea have no interference towards cholesterol determination. The limit of detection of cholesterol in synthetic cerebrospinal fluid is calculated to be 7.76 × 10-8 M.
Figure 8: (A) Overlay of cyclic voltammograms at scan rate 0.05 Vs-1 and (B) Overlay of square wave voltammograms of Cholesterol (1.64 × 10-3 M to 1.18 × 10-2 M) in synthetic cerebrospinal fluid at Pt/L@/CTAB/AgNO3 electrode. (C) Current vs. concentration of cholesterol (1.64 × 10-3 M to 1.18 × 10-2 M) plot from square wave voltammetry in synthetic cerebrospinal fluid at L@Pt/CTAB/AgNO3 electrode. RE: Ag/AgCl (3 M NaCl).
Conclusion
Lawsone encapsulated CTAB modified platinum disc electrode when coated with AgNO3 can detect cholesterol by voltammetry and impedance spectroscopy. The voltammetric currents and charge transfer resistance decrease with cholesterol concentration. The ethanol-water solution of lawsone with AgNO3 found to show fluorescence enhancement with cholesterol. The voltammetric sensing of cholesterol was also proved in artificial cerebrospinal solution. The limit of detection of cholesterol in 0.1 M KCl is 4.0 × 10-7 M and 7.76 × 10-8 M in synthetic cerebrospinal fluid.
Acknowledgement
The authors thank DST, New Delhi for the MRP under SERB No. EMR/2016/001745 and ASTEC, Guwahati for financial support. DST is also thanked for financial support to the department of Chemistry, GU through FIST program.
References
- Kurihara K, Ohto K, Tanaka Y, et al. J Am Chem Soc.1991;113: 444-450.
- Aoyama Y, Yamaguchi A, Asagawa M, et al. J Am Chem Soc. 1988;110:4076-4077.
- Hamilton AD, Engen DV. J Am Chem Soc.1987;109:5035-5036.
- Aoyama Y, Tanaka Y, Sugahara S. J Am Chem Soc.1989;111:5397-5404.
- Manisankar P, Selvanathan G, Vedhi C. Talanta. 2006;68:686-692.
- Ansari MZ, Cho C. Sensors. 2008;8:7530-7544.
- Petrova SA, Kolodyazhny MV, Ksenzhek OS. J Electroanal Chem.1990;277:189-196.
- Chethana BK, Basavanna S, ArthobaNaik Y. J Anal Chem. 2014;69:887-891.
- Hijji YM, Barare B, Zhang Y. Sens Actuators B Chem. 2012;169:106-112.
- Dryhurst G. Academic Press. 1977.
- Sonawane M, Tayade K, Sahoo SK, et al. J Coord Chem. 2016;69:2785-2792.
- Vire JC, Patriarche GJ, Christian GD. Anal Chem. 1979;51:752-757.
- Vire JC, El Maali NA, Patriarche GJ, Christian GD. Talanta. 1988;35:997-1000.
- Guin PS, Das S, Mandal PC. Int J Electrochem. 2011:1-22.
- Narwal V, Deswal R, Batra B, et al. Steroids. 2019;143:6-17.
- Alagappan M, Immanuel S, Sivasubramanian R, et al. Arab J Chem. 2020;13:2001-2010.
- Zaheer Z, Danish EY, Kosa SA. Chin J Chem Eng. 2021;32:212-223.
- Razavian MH, Masaimanesh M. Nanomed J. 2014;1:339-345.
- de Toledo RA, Santos MC, ETG Cavalheiro ETG, et al. Anal Bioanal Chem. 2005;381:1161-1166.
- Lamari AS, Fattouh A, Qouatli SE, et al. Acta Tech Corvin. 2013;11:39-42.