Reviews: 2022 Vol: 14 Issue: 6
Antibacterial Activity of Some Raw Clays from Congo Brazzaville
Rarahu Nursie Mboho Oba1*, Cyr Jonas Morabandza CJ2, Zita Flora Mpissi Diamouangana1, Ronel Loubaki2, Tarcisse Baloki, Gustia Mize1, Joseph-Marie Moutou2
1Department of Applied Inorganic Chemistry, Ecole Normale superieure, Universite Marien Ngouabi, Congo Brazzaville
2Department of Cellular and Molecular Biology, Marien Ngouabi University, Congo, Brazzaville
- Corresponding Author:
- Rarahu Nursie Mboho Oba
Department of Applied Inorganic Chemistry,
Ecole Normale superieure,
Universite Marien Ngouabi,
Congo Brazzaville
Received: 02-May-2022, Manuscript No. JOCPR-22-47398; Editor assigned: 04-May-2022, PreQC No.JOCPR-22-47398 (PQ); Reviewed: 17-May-2022, QC No. JOCPR-22-47398; Revised: 02-jun-2022, Manuscript No.JOCPR-22-47398(R); Published: 16-jun-2022,DOI:10.37532/0975-7384.2022.14(6).032.
Abstract
This work evaluates the antibacterial activity of some raw clays from Congolese a localities: Loukolela, Loutete, Missafou, Dolisie and Dongou. Bacterial sensitivity tests were performed on Escherichia coli, staphylococcus aureus and Pseudomonas aeruginosa strains. The raw clays were put in contact with 3 Strains. The results obtained show that all the raw clays simulate the growth of E.coli and P. aeruginosa whereas, that of S. aureus is stimulated essentially by the clays of Loukolela, Loutete and Missafou.
Keywords
Clay; E.coli; P. aeruginosa; S. aureus, Antibacterial
Introduction
Clay minerals are used therapeutically in the treatment of bacterial infections [1, 2]. It has been proved that the most important way to control diseases caused by bacteria is antibiotic treatment, however, the abuse or overuse of antibiotics causes various side effects such as antibiotic resistance in the body [3, 4]. It is then necessary to develop new types of antibacterial agents as food additives [5]. Some in vitro studies have shown that clays protect the intestinal mucosal barrier and absorb toxins, bacterial and viruses [6]. The adsorption capacity of clays is a function of the clay species, 2:1 clays adsorb more than 1:1 clays. In Congo Brazzaville local people in clay areas use clay as a remedy for the treatment of diarrhoea and pregnant women consume it to eliminate nausea caused by pregnancy. Clays are interesting because they may reveal an antibacterial mechanism, which could provide an inexpensive treatment for bacterial and skin infections, Worldwide [7]. The abundance of clays in Congo Brazzaville would make a low cost for a treatment against bacterial infections. This study is conducted to evaluate the antibacterial activity of raw clays from Congo Brazzaville.
Materials and Methods
The materials used were taken from five localities in Congo-Brazzaville, namely: Loukolela, Loutete, Missafou, Dongou and Dolisie. All these clays were subjected to physicochemical and mineralogical characterization (Table 1) [8-10].
Table 1: Clay species and origin
| Localities | Predominant mineral | Type |
|---|---|---|
| Loukolela | Montmorillonite | 2:1 |
| Missafou | Talc | 2:1 |
| Loutete | Kaolinite | 1:1 |
| Dolisie | Kaolinite and illite | 1:1 and 2:1 |
| Dongou | Montmorillonite and kaolinite | 2:1 and 1:1 |
Clinicat bacterial strains tested
The strains used were is isolated from patients at the National public health laboratory in Brazzaville and then stored in liquid LB medium plus glycerol in ependof tubes in the refrigerator at -4°C in the laboratory. These strains consisted of two gram-negative bacteria: Escherichia coli clinical bacteria and Pseudomonas aeruginosa and a gram-positive bacterium staphylococcus aureus.
Culture media used: The media used are liquid LB medium, Mannitol salt agar, cetrimide agar, Methylene blue eosin and physiological water with 9% NaCl.
Test for the presence of germs in clays: A quantity of 7 g of each raw as well as treated unsterilized clay was introduced into five tubes each containing 10 ml of physiological water sterilized at a temperature of 121°C in the autoclave for 15 minutes. 0.1 ml volume of each mixture was plated on agar specific for pseudomonas aeruginosa, Staphylococcus aureus and Escherichia Coli. These cultures were incubated in the oven at a temperature of 37°C for 24 hours.
Microbial sensitivity testing: Three bacterial suspensions containing E.coli, S. aureus and P.aeruginosa strains respectively with a density of 106 CFU/ml on the M c Farland scale were prepared. 7 g of clay previously sterilized in an autoclave at 121°C were mixed with 10 ml of each of the suspensions. The tubes of mixture thus obtained were incubated after homogenization at 37°C for 24 h. Successive dilutions up to 10-10 in physiological water were made after incubation for 24 h. A viable colony count was performed and the TSV calculated from the Kra formula [11].
TSV= NA/NT × 100 With NA: number of colonies of the clay-bacteria mixture.
NT: number of colonies in the control.
X-Ray Fluorescence Analysis: The chemical analysis was carried out by x-ray fluorescence using a philips X-ray generator. The loss of water was carried out by x-ray fluorescence using a philips x-ray technique is based on the interaction of X-ray radiation with electrons. These interactions lead to the internal reorganization of the electrons, responsible for the emission of a characteristic radiation, which will allow identifying the studies atoms (qualitative aspect). The measurement of the intensity of the characteristic lines will allow to identify the atoms and to determine the centesimal composition of the analysed sample.
Results and Discussion
The Table 2 presents the results of the chemical analysis by XRF of the raw clays. It shows that the five clays (Louk, tout, Miss, Doli and Dong) are mainly composed of silica with percentages of 52,96; 60,04; 64,56; 65,93; 55,77 respectively, iron ores with percentages of 7,32 for Louk, 6,00 for Miss, 7,74 for Lout, 3,89 for Dolisie and 3,93 for Dongou and aluminium oxides with 19,05 for Louk, 13,12 for Miss, 30,43 for Lout, 21,76 for Doli, 34,29 for Dong. These results suggest that silica, iron and aluminium ores predominate in the five types of Congolese clays. However, these clays show several differences in their compositions. The percentage of MgO is higher in the Missafou clay than in the others remember that the characterization of miss has revealed an abundance of talc as a clay species. The octahedral layers in lalc are constituted of Mg2+. Loukolela, Missafou, Dolisie and Dongou on the other hand present a high percentage of iron. We also note that the clays of Loukolela and Dongou have a lower percentage of silica compared to the others.
Germ Test in Raw Clays
The Table 3 shows the results of the verification test of the presence or not of germs in the different raw clays. The results obtained reveal the presence of Staphylococcus aureus and Escherichia coli Strains in the clays of Loukolela, Missafou and Loutete while they are totally absent in those of Dolisie and Dongou. The absence of the Pseudomonas aeruginosa strain is noted in all the clays. These results show that it is necessary to sterilize these clays before any use.
Table 2:Chemical analysis by X-ray fluorescence of raw clays
| Sample | SiO2 | Al2O3 | Fe2O3 | MnO | MgO | CaO | K2O | Na2O | TiO2 | P2O5 |
|---|---|---|---|---|---|---|---|---|---|---|
| Loukolela | 52,96 | 19,05 | 7,32 | 0,38 | 2,368 | 1,87 | 1,67 | 0,546 | 0,72 | 0,21 |
| Missafou | 60,04 | 13,12 | 6,00 | 0,005 | 6,20 | 0,20 | 1,26 | 0,450 | 0,54 | 0,06 |
| Loutete | 64,56 | 30,43 | 7,74 | 0,006 | 0,30 | 0,11 | 0,62 | 0,51 | 1,68 | 0,07 |
| Dolisie | 65,93 | 21,76 | 3,89 | 13 | 1,16 | 0,07 | 3,16 | 0,44 | 1,12 | 0,10 |
| Dongou | 55,77 | 34,29 | 3,93 | 0,34 | 0,47 | 0,08 | 1,066 | 1,23 | 2,48 | 0,07 |
Table 3: Germ test in raw clays
| Locality/Strain | Loukolela | Missafou | Loutete | Dolisie | Dongou |
|---|---|---|---|---|---|
| Staphylococcus aureus | + | + | + | - | - |
| Escherichia coli | + | + | + | - | - |
| Pseudomonas aeruginosa | - | - | - | - | - |
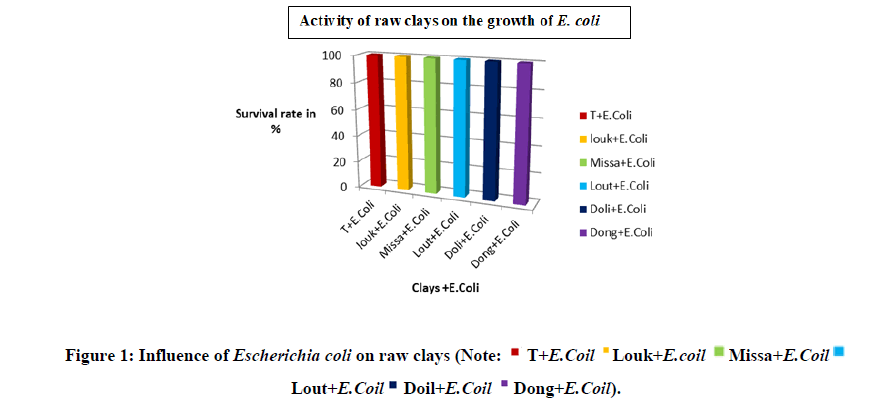
The Figure 1 shows the activity of raw clays on the growth of Escherichia coli. It can be seen that raw clays (Louk, Miss, Lout, Doli, and Dong) stimulate the growth of E. coli species with a survival rate of 100%. This could be explained by the effect that some microbes in contact with clay minerals draw energy necessary for their growth [12].These results are close to those kouakou whose clays AK1, AK2, UB1 stimulated the growth of E. coli.
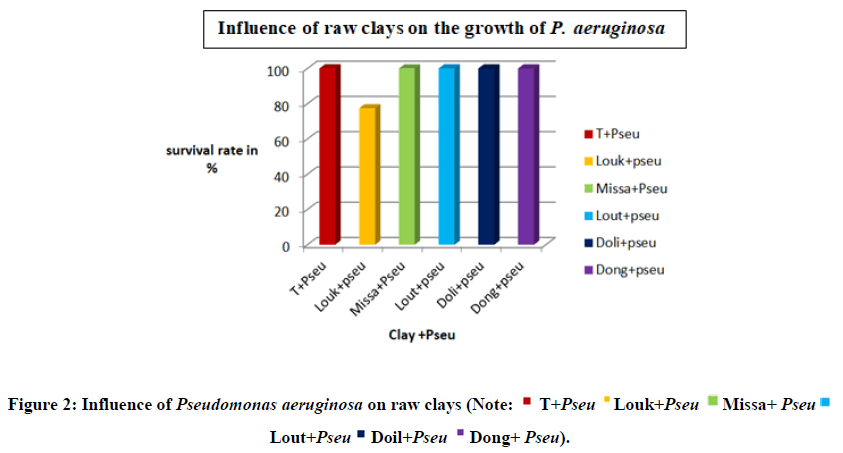
The Figure 2 shows the influence of raw clays on the growth of P. aeruginosa. A survival rate of 100% is observed, indicating a possible stimulation of growth. While in the Loukolela clay this growth is lower compared to the clays in other localities. This activity can be explained by the dominant presence of montmorillonite in Louk [13].The literature reports that pure and unmodified montmorillonite has no effect against bacteria. A slight decrease in absorbance and number of colonies can be exolained by the adsorption proprties of the material [14], recall that the characterization of loukolela clay has shown that the montmorillonite of this clay is of the calcic type, it has been reported in the literature that the Ca2+ is an essential nutrient for the growth of bacteria [15] since the Ca2+ in the clay is sequestered in the interfoliar space which favours the sloving down of Pseudomonas with the clay and also being a swelling clay, the clay would absorb a large quantity of water from the medium which is unfavourable to the growth of Pseudomonas aeruginosa. This result contrasts with that of kouakou where all clays inhibited the growth of pseudomonas aeruginosa species.
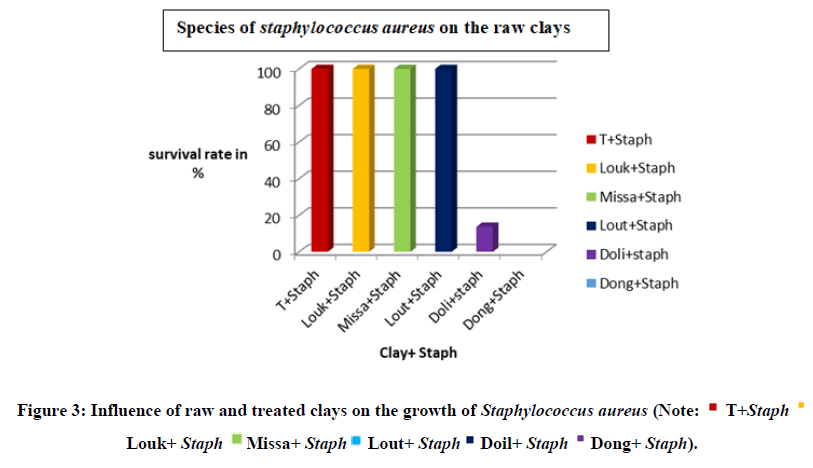
The Figure 3 shows the influence of the species of staphylococcus aureus on the raw clays. We note that that the Dongou clay has an inhibitory activity with a survival rate of 0% translating a bactericidal effect and that of Dolisie has a survival raye of (13,66%) showing a bacteriostatic effect on Staphylococcus aureus. The clays of Loukolela, Loutete and Missafou stimulate the growth of bacteria. This result is close to that of Kouakou. The strong inhibition observed would certainly come from the presence of illite, montmorillonite, kaolinite in Dongou clay and the combined action of these minerals could induce a decrease in the amount of water in the environment, unfavorable to the development of the species Staphyloccocus aureus by the type of preponderant minerals and the different combinations.
Conclusion
This work evaluates the antibacterial activity of the raw Congolese clays, it emerges from this study that the raw clays of Loukolela, Loutete, Missafou, Dolisie and Dongou are rich in iron, silicon, aluminium, these raw clays stimulate the growth of Escherichia coli, Pseudomonasaeruginosa aureus. Raw Dolisie and Dongou clays inhibit the growth by showing a bactericidal effect. These results show that some of these clays present an antibacterial activity however this activity can be improved by eliminating certain elements favorable to the development of bacteria. It has been recognized in the literature that the influence of clay minerals on microbial physiological and biochemical properties could both promote and inhibit growth, depending on the microbial type and varying from one species of microorganisms to another. We assume that the physicochemical proprties of clays indirectly kill bacteria by producing an unfavorable environment for bacterial growth. These results open a perspective in the use of clays against bacterial infections.
Conflicts of Interest
The author/s declares that they have no conflict of interest.
References
- Fongnzossie EF, Tize Z, Nde PF, et al. South African J Bot. 2017;112:29-39.
- Patil SB, Palled MS, Bhat AR. Asi J Res Chem. 2010;3:685-687.
- Ivce R, Sabalja Ð, Zuskin S. Int Symposium ELMAR (ELMAR) 2015; 15:193-196.
- Kunin CM. Annal Internal Med. 1993; 118:557-561.
- Dong W, Lu Y, Wang W, et al. Chem Eng J. 2020;382:122984.
- Carretero MI, Gomes CS, Tateo F. Develop Clay Sci. 2006;1:717-741.
- Haydel SE, Remenih CM, Williams LB. J Antimicrob Chemother. 2008;61:353-361.
- Moutou J, Mbedi R, Elimbi A, et al. Res J Environment Earth Sci. 2012;4:316-324.
- Mandimba GR, Mondibaye A. Biolog Agricult Horticul. 1996;13:197-204.
- Oba RN, Ifo GM, Madila EE, et al. J Minerals Material Characteriz Engineer. 2022;10:93-105.
- Photos-Jones E, Keane C, Jones AX, et al. J Archaeolog Sci. 2015;57:257-267.
- Morrison KD, Misra R, Williams LB. Sci Rep. 2016;6:1-3.
- Bartusek S, Skacel A. Int Multidisciplin Sci GeoCon: SGEM. 2018;18:81-87.
- Williams LB, Haydel SE, Giese RF, et al. Clays Clay Min. 2008;56:437-452.
- Brennan FP, Moynihan E, Griffiths BS, et al. Sci Total Environment. 2014;468:302-305.