Original Articles: 2025 Vol: 17 Issue: 1
Anti-Alzheimer and Other Neuroprotective Activity of Glitazones on PPAR-γ Receptors: An Update
Rajiv Kumar1*, Ankush Goyal2, Lalita Dahiya2, Arvinder Pal Singh3, Isha Kapila1
1Department of Pharmacy, Chandigarh College of Pharmacy, Landran-140307, Punjab, India
2Department of Pharmacy, Institute of Pharmaceutical Sciences, Kurukshetra University, Kurukshetra-136119 India
3Department of Pharmacy, Maharaja Agrasen University, Kalujhanda-174103, Himachal Pradesh, India
*Corresponding Author:
Received: 22-Nov-2023, Manuscript No. JOCPR-23-120774; Editor assigned: 25-Nov-2023, PreQC No. JOCPR-23-120774 (PQ); Reviewed: 09-Dec-2023, QC No. JOCPR-23-120774; Revised: 09-Jan-2025, Manuscript No. JOCPR-23-120774 (R); Published: 16-Jan-2025, DOI:10.37532/0975-7384.2025.17(1).143.
Citation: Rajiv K, et al. 2025. Anti-Alzheimer and Other Neuroprotective Activity of Glitazones on PPAR-γ Receptors: An Update. J. Chem. Pharm. Res., 17:143.
Copyright: © 2025 Rajiv K, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited
Abstract
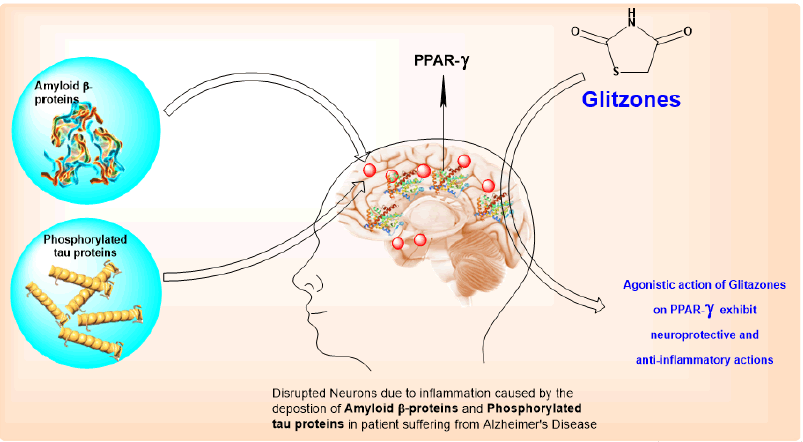
Alzheimer's disease is identified by the deposition of amyloid β-proteins extracellularly and phosphorylated tau proteins intracellularly in the grey matter of the brain. The formation of amyloid plaques leads to the inflammatory response in the region of the entorhinal cortex, hippocampus, and cerebral cortex. Agonisticaction on PPAR-γ has been reported to possess anti-inflammatory and neuroprotective functions. Glitazone is a versatile molecule with PPAR-γ agonistic activity which leads to neuroprotective and anti-inflammatory actions. Biological activities and the low cost of glitazone attracted scientists to explore novel clinical uses of the drug. This led to different clinical investigations of glitazones in animals and humans in the last decade. In this review, authors presented glitazones ongoing development and potency as neuroprotective and anti-inflammatory agents in Alzheimer disease with reported mechanisms. The review will help develop new clinical investigations of glitazones as Anti-alzheimer and other cognitive and neurodegenerative disorders.
Keywords
Alzheimer's disease, Neuroprotective, Nootropic, Parkinsonism, Anti-inflammatory, 2,4-thiazolidinediones, Glitazones
Introduction
Alzheimer's Disease (AD) is caused by the accumulation of amyloid beta proteins extracellularly and phosphorylated tau proteins intracellularly in the grey matter of the brain which leads to the formation of amyloid plaques leads to the inflammatory response in the region of entorhinal cortex, hippocampus, and cerebral cortex. The nuclear receptors peroxisome proliferator-activated receptors are ligand-activated transcription factor which plays an important role in the regulation of glucose and lipid metabolism and inhibits inflammatory gene expression. Agonists of PPAR-γ can be used as an anti-AD drug. There are several pieces of evidence of the potency of PPAR-γ agonists in improving inflammation-related conditions and enhancing memory in Alzheimer’s models. Researchers have proved that PPAR-γ inhibits the formation of inflammatory cytokines and thus improves inflammatory conditions due to the aggregation of amyloid beta proteins and phosphorylated tau proteins. Thus, the anti-Alzheimer activity of Pioglitazone and Rosiglitazone is reviewed here (Figure 1) [1].
Alzheimer's disease is a CNS disease which slowly affects memory and thinking skills and the ability to carry out routine tasks. In most people with the disease-those with the late-onset type- symptoms appear in old age of mid 60’s. Alzheimer’s disease is characterized by the loss of neurons and synapses in the cerebral cortex and certain subcortical regions. This loss results in gross atrophy of the affected regions, including degeneration in the temporal lobe and parietal lobe and parts of the frontal cortex and cingulated gyrus. AD is classified into two types, one is genetic which is known as Familial Alzheimer’s Disease (FAD) and the second is Sporadic Alzheimer’s Disease (SAD). SAD is not considered genetic as it is due to senility conditions. FAD represents only 4-8% of cases of Alzheimer’s while SAD represents most of the cases of Alzheimer’s [1].
Functional MRI has shown changes in neuronal network activities in AD patients and people at risk of AD. It was observed that abnormal activity and connectivity in the AD patient were enough to distinguish from normal activity and connectivity in volunteers without AD.
Experiments suggest that AD is not only responsible for the inactivation of neuronal networks but also causing abnormal activities in the neuronal network which alters learning, memory, and other cognitive functions. As over-activity in the neuronal network was also found, this may be considered responsible for neurodegeneration and also for increased incidences of epileptic seizures [2].
Literature Review
Glitazones
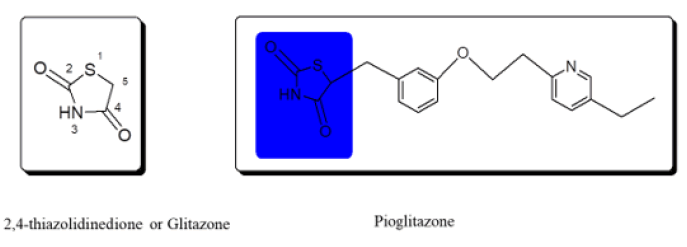
2,4-Thiazolidinedione derivatives were launched as antidiabetics in the 1990’s. Later the derivatives of 2,4-thiazolidinedione were removed due to hepatotoxicity. Today, many researchers have synthesized novel derivatives and shown uses for 2,4-thiazolidinedione compounds. 2,4-Thiazolidinedione (2,4-TZD) is a five-membered pentacyclic heterocyclic ring containing sulphur, nitrogen, and carbon as the members of the ring system while possessing two ketonic groups at 2nd and 4th positions as the substituents. The carbonyl group of 2,4-thiazolidinedione is highly stable. Pioglitazone and Rosiglitazone are the oldest drugs belonging to glitazones.
Several research papers proved the vast range of potency of 2,4-TZD as an antimicrobial, anticancer, antiarthritic, anti-inflammatory, antioxidant, and other miscellaneous therapeutic activities other than antidiabetic activity (Figure 2).
Aetiology of AD
The main accumulation of two proteins is responsible for AD which is Amyloid Beta protein extracellularly and Tau protein intracellularly in the grey matter of the nervous system. The main pathological hallmarks of Alzheimer’s disease include extracellular deposition of β-amyloid plaques, and intra-neuronal neurofibrillary tangles comprising filaments of phosphorylated tau proteins. Loss of cortical cholinergic neurons in AD probably accounts for memory impairment.
Science so far
Monocyte and lymphocyte cytokine gene and protein expression of interleukin (IL)-6 were found to be toa larger extent in diabetic patients than in normal volunteers. After six months under observation, patients taking Pioglitazone showed lower levels of monocyte gene and protein expression of IL-1β, IL-6, and IL-8 (and IL-2, IL-6 and IL-8 from lymphocytes). Also, IL-6, IL-8 and MCP-1 gene expression were found to be decreased significantly by 50% in human adipocytes in patients taking Pioglitazone. This experiment demonstrates that pioglitazone therapy in diabetic patients can reduce pro-inflammatory gene and protein expression from both monocytes and lymphocytes [3].
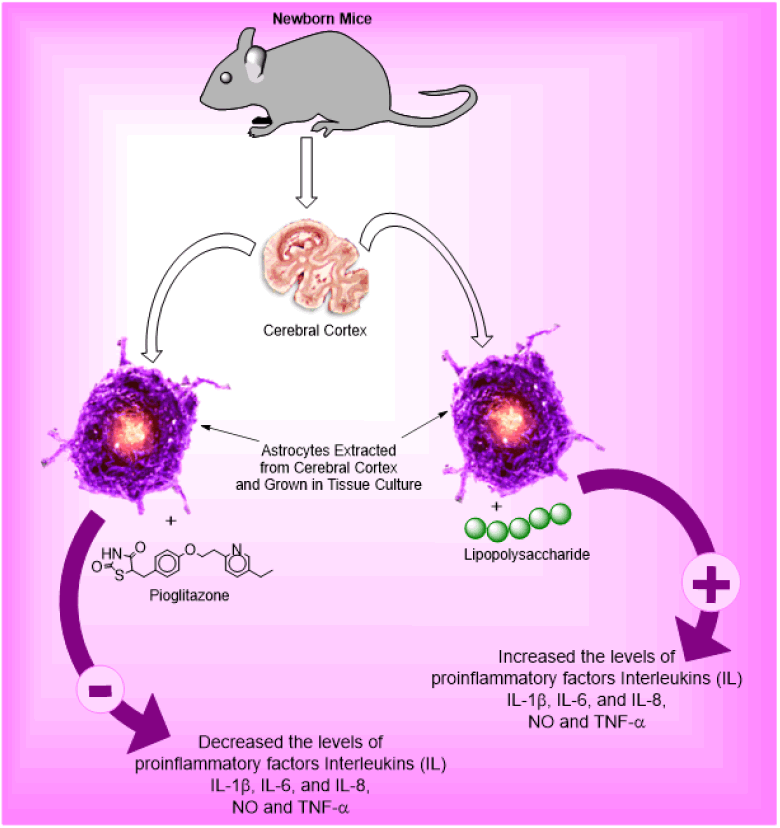
Qiu D, et al. explore the effects of Pioglitazone (PIO) on cytokine and chemokines secreted in astrocytes (star-shaped glial cells). Astrocytes are extracted from the cerebral cortex of newborn mice and grown in tissue culture and inflammation is caused by Lipopolysaccharides (LPS). Inflammation caused by LPS significantly increased the levels of pro-inflammatory factors Interleukin (IL)-1β, IL-6, and IL-8, but decreased the level of anti-inflammatory factors IL-4 and IL-10 (p<0.05) compared to untreated control astrocytes. Also, the levels of Nitric Oxide (NO) and Tumour Necrosis Factor (TNF)-α are increased significantly. PIO treatment in LPS-stimulated astrocytes had the opposite effect, inhibiting the secretion of NO, TNF-α, IL-1β, IL-6, and IL-8 and enhancing IL-4 and IL-10 secretion (p<0.05). In addition, PIO treatment suppressed the expression of pro-inflammatory chemokines Ccl20, MCP-1, and MIP-1α mRNA in astrocytes stimulated with LPS (p<0.05). This research proved that PIO inhibits the secretion of neuro-inflammatory agents i.e., cytokine and chemokine from astrocytes (Figure 3) [4].
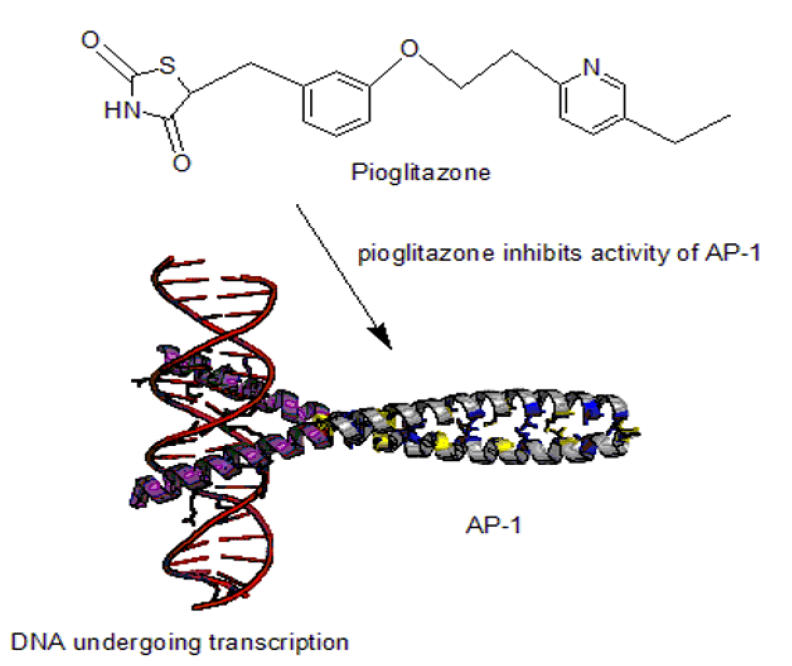
Wu Chun-Hsien, et al., performed experiments for evaluating the anti-inflammatory activity of Pioglitazone (PIO) in Atherosclerosis. It was demonstrated that pioglitazone inhibited the production of IL-2, IL4, IFN-, and TNF- from activated T cells. Molecular investigation indicated that pioglitazone downregulated the activity of AP-1 DNA binding protein in stimulated T lymphocytes. In this in-vitro study, pioglitazone might have had some anti-inflammatory effects on human peripheral T lymphocytes via regulation of both cytokine production and AP-1 DNA binding activity (Figure 4) [5].
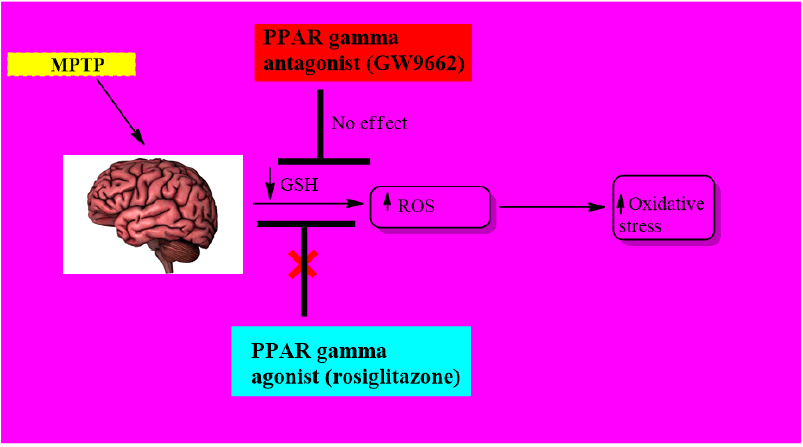
Heather LM, et al., have performed in vitro study of the protective effect of PPAR-γ agonist, rosiglitazone and PPAR-γantagonist GW9662 in MPP+ or MPTP-induced reactive oxygen species in Parkinson’s disease. It has been found that only rosiglitazone has a protective effect by reducing oxidative stress with an upregulation of glutathione-S-transferase but maybe without causing PPARγ activation. While for in vivo study on PPARγ levels in SNpc, western blot and qPCR analysis was evaluated. Results have shown that PPARγ levels are overexpressed 7 days after MPTP treatment. To confirm PPARγ activity in neuronal survival within SNpc, mice were treated with GW9662 and evidence supported that antagonism leads to neuronal loss (Figure 5) [6].
Xu S et al. Morris water maze tests, ELISA and electrophysiological studies on injecting Aβ42-oligomer to rat model showed that treatment with rosiglitazone inhibits astrocytes and microglial activation. Inhibition of microglial activation helps prevent Aβ42-oligomer induced memory impairment by significantly decreasing levels of interleukin-1beta (IL-1β) and interferon-gamma (IFNγ). These observations suggest that pioglitazone’s suppressive action on neuro-inflammation is related to its mechanism of action in treating Aβ42-oligomer induced memory impairment [7].
Machado MMF et al., in another study, a 6-hydroxydopamine model of PD was treated with others. PPAR-agonist, pioglitazone showed similar results, for example, injection of pioglitazone (30 mg/kg) directly into male Wistar rats for 5 days receiving aninfusion of 6-hydroxydopamine decreases microglial activation and nuclear factor (NF-κB) Evidence obtained from this model of PD in Substantia Nigra (SN) through western blot analysis, indicated that pioglitazone has potential role in managing neuro-inflammation by inhibiting pathological biomarkers [8].
Justin A, et al. had synthesized and evaluated the neuroprotective effect of two glitazones targeting PPAR-dependent PGC-1α receptors. In context with design, firstly bioisosteres of glitazones were synthesized followed by performing docking studies in order to analyze the structural changes which are likely to occur during binding with active site. For evaluation part, Lipopolysaccharide (LPS) intoxicated SH-SY5Y neuroblastoma cells were selected. Glitazones were found to have neuroprotective effect by decreasing levels of LPO, NO, TNF-a, and IL-1b and increasing levels of antioxidant enzymes such as SOD, CAT, and it has been concluded that among two glitazones, G2 has shown promising results as compared to G1 and standard glitazones.
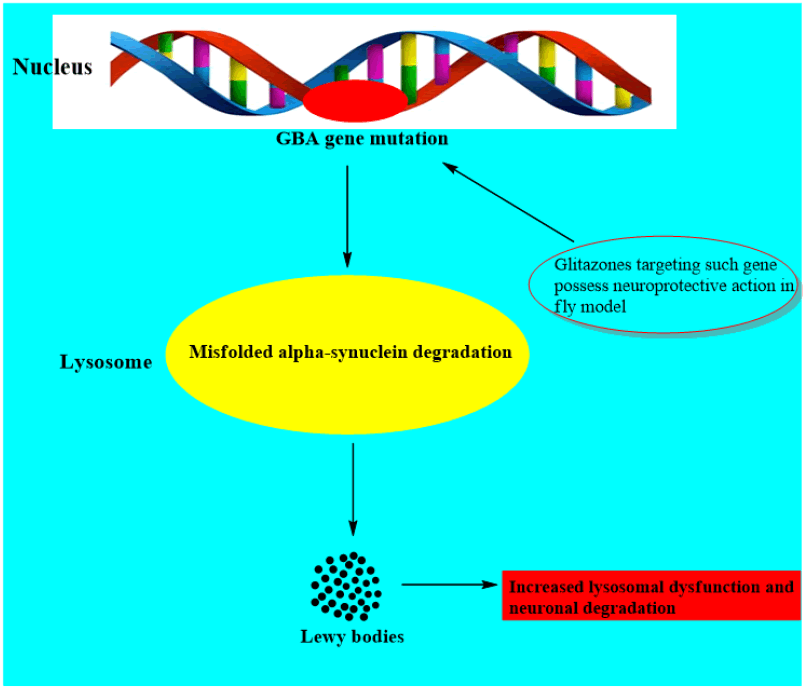
It has been investigated that one of the strongest causative factors linked with PD is the mutation in glucocerebrosidase gene (GBA1 linked). However the pathological mechanism between the defect in this gene and PD is uncertain (Figure 6) [9].
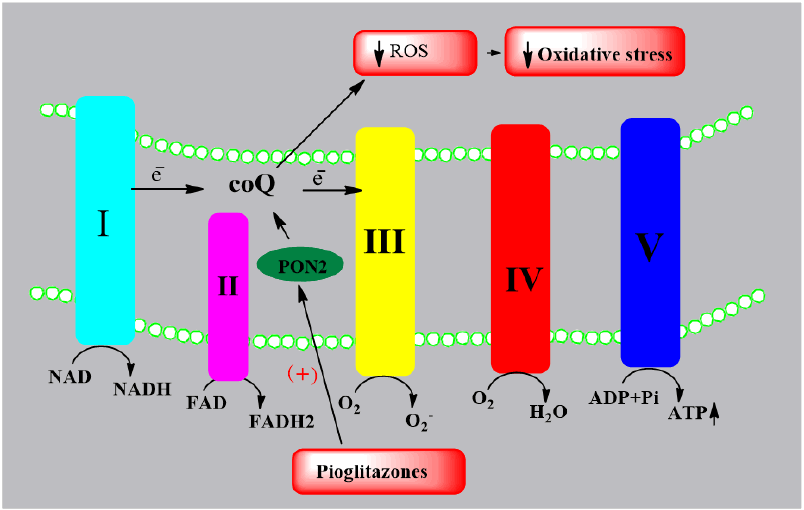
Blackburn JK, et al., evaluated the effect of PGZ on paraxonase-2(PON2) expression in animal model of Parkinson’s disease. PON2 is in inner mitochondrial membrane which increases the function of coenzyme Q in Electron transport chain, thereby reducing oxidative stress. In this study, oral pioglitazone was given to male green monkeys in a dose of 5 mg/kg/day for one and three weeks. Results suggested that pioglitazone enhances PON2 expression in substantia nigra, striatum hippocampus and dorsolateral prefrontal cortex as compared to control for following one week but by three weeks of treatment, expression gets decreased (Figure 7) [10].
Further studies by Hassanzadeh K, et al. evaluated the role of lobeglitazone on motor function in rat co-model of Diabetes-PD. Firstly 48 male Wistar rats were injected with a single dose of streptomycin, rotenone and high calorie diet for 60 days followed by different doses (0.1,0.2 or 1 mg/Kg) of lobeglitazone. These studies indicated that lobeglitazone with concentration of 1 mg/kg showed a significant reduction in tyrosine hydroxylase level but there is increase of TNF-α and NF-κB levels in SN and striatum using ELISA. Likewise changes in levels of PPAR- receptor were also evaluated and results indicated that lobeglitazone has possibility to have neuroprotective effect in patients with diabetes and PD both [11].
A new emerging candidate, leriglitazone which has ability to cross blood brain barrier is of great interest for researchers to treat neurodegenerative diseases. In recent studies shown by Rodríguez-Pascau. et al. Leriglitazone has shown multiple effects on frataxin-deficient cellular and animal models of Friedreich-ataxia. In frataxin deficient dorsal root ganglia neurons, it has found to cause an increase in 48% of frataxin levels at concentration of 500 nM, as evidenced by western blot analysis. Moreover, treatment with leriglitazone for 5 days at same concentration had decreased neurite degeneration, increased cell survival and membrane potential thereby affecting Na+/Ca2+ exchanger. Furthermore, when neonatal rat ventricular cardiomycetes were treated with leriglitazone at concentration of 500 nM for 7 days, results showed that there was 42.3% decrease in lipid droplets accumulation indicating its preventing effect. Leriglitazone significantly improved motor function in animal model of YG8SR mice at concentration of 0.06% (50 mg/kg/day) as evaluated by balance beam team test. Additionally, it has shown protective action in FRDA models by increasing PGC-1α, GRP75 and frataxin levels in both control and FRDA patient cells [12].
Regarding neuroprotective effect of glitazones in epilepsy, there were studies on mice treated with rosiglitazone in pilocarpine induced status epilepticus. Results from these studies showed that rosiglitazone treatment significantly reduced the activation of microglia and expression of iNOS in microglia. Additionally, immunofluorescence staining indicated that rosiglitazone prevents neuronal loss in temporal lobe, DG regions and CA1 hippocampi as compared to control group. Furthermore, administration of rosiglitazone in SE mouse brain decreased CD86 expression and increased CD206, Arg-1 expression in microglia in forebrain as determined by flow cytometry. However, it did not significantly change mRNA expression of pro-inflammatory cytokines in forebrain and hippocampiregions among all three groups as evidenced by RT-PCR analysis. These studies indicate that rosiglitazone induced polarization of microglia but inhibition of inflammatory response in brain is not involved in protecting against pilocarpine induced status epilepticus.
Justin, Ashwini, et al., performed an experiment using two glitazone derivatives which have been proved potent on PPAR-γ receptors by docking studies. First, oral toxicity study was done for glitazones in rats to determine toxicity profiling and therapeutic range of neuroprotective action. Pioglitazone was used as standard. Before induction of neuro-inflammation, rats were treated with glitazone derivatives and Pioglitazone for four consecutive days. Neuro-inflammation was induced at the fifth day by Intra-Cerebro Ventricular (ICV) administration of lipopolysaccharides (LPS) (2 mg/ml) using stereotaxic apparatus. After 7 days, the behavior of rats was assessed by neuro biochemical evaluation. Rats those were pre-treated with two glitazone derivatives menifested significant reversal of the behavior symptoms. Glitazones have shown significant reduction in the levels of LPO, NO, TNF-α, IL-1b and also increased the levels of antioxidant enzymes such as SOD (Superoxide dismutase), CAT (Catalase), and GSH (Glutathione) in the brain of LPS-administered rats. Glitazone derivatives shown significant effects when compared to standard Pioglitazone in neuroprotection. It was supposed that PPAR-γ induced improvements of cytokines was responsible for neuroprotection and reversal of behavioural symptoms [13].
Wang, Zhao, et al. had reported that Parkinsonism was caused in models using both in vitro and in vivo MPP+/MPTP. In study, significant improvement was observed in treatment with pioglitazone with increased levels of Tyrosine hydroxylase-1 levels. Pioglitazone also increases the expression of PPAR-g and increased the number of mitochondria along with improvement in structure of mitochondria. From in vitro studies, 2,4-thiazolidinedione resulted in increased levels of molecules regulated function of mitochondria, including PGC-1, nuclear respiratory factor 1 (NRF1), NRF2, and mitochondria fusion 2 (Mfn2), and inhibited mitochondria fission 1 (Fis1). Levels of B-cell lymphoma 2 and ERK proteins were found to be increased in models treated with 2,4-thiazolidinedione derivatives [14].
Zhao Lützen, et al. Pioglitazone was administered in models 5 days before and 24-48 hr after alleviation of neurological impairments by MCAO (Experimental Middle Cerebral Artery Ischemia and Functional Recovery). In primary cortical neurons, glutamate induced release of lactate dehydrogenase is decreased by pioglitazone. This protective effect was reversed by co-treatment with PI3K and Akt inhibitors. Pioglitazone increases the expression of anti-oxidative transcription factor NRF2 and its target gene protein, heme oxidase-1, in the peri-infarct area. Pioglitazone was also found to enhance the levels of Antioxidant Response Element (ARE) in neuronal PC12 cells. It was demonstrated that neuroprotective effects of PPAR-g in peri-infarct brain tissues is because of activation of PI3K/Akt and Nrf2/ARE pathways.
Thal Heinemann, et al. proved that 2,4-TZD effectively decreases the inflammation and brain lesions by activation of PPAR-γ. Their study demonstrates the anti-inflammatory effect of pioglitazone and rosiglitazone on primary and secondary damage of brain after experimental traumatic brain injury. A regulated impact injury was caused in cortical area of mice brainm RNA expression, PPAR-γ target genes (LPL, GLT1 and IRAP/Lnpep) and inflammatory markers (TNF-α, IL-1β, IL-6, and iNOS) were measured at 15 min, 3 h, 6 h, 12 h, and 24 h post-trauma. Pioglitazone reduced the histological damage and inflammation in a dose-dependent fashion. In contrast, rosiglitazone failed to suppress inflammation and histological damage. PPAR-γ and PPAR-γ target gene expression was not induced by pioglitazone and rosiglitazone. The result proved that anti-inflammatory effect of Pioglitazone is not only PPAR-γ dependant [15].
Swanson Joers, et al. said that inflammation is caused in the brain of Rhesus monkeys by single intra-carotid injection of 20 ml of saline containing 3 mg of neurotoxin MPTP. After 24 hrs the monkeys were divided into three categories based on severity of symptoms and treated with pioglitazone with dose 0-5mg/kg. After three months of daily doses, animals were necropsied. Significant improvements observed in monkeys treated with dose 5mg/kg as compare to that treated with zero dose of pioglitazone. Behavioral improvements were associated with preservation of nigrostriatal dopaminergic markers observed as higher Tyrosine Hydroxylase (TH) putaminal optical density. Pioglitazone treated monkeys also showed a dose-dependent modulation of CD68-ir inflammatory cells that was decreased for 5 mg/kg treated animals compared to placebo. This research can conclude that oral administration of pioglitazone as neuroprotective showed significant results in improvement in symptoms of Parkinsonism in Rhesus monkey and supports the concept that PPAR-γ is a viable target against neurodegeneration [16].
To Ribe, et al. performed experimentfor impaired insulin signalling is increasing more notions to make contributions to Alzheimer’s ailment/disease (AD). The e4 isoform of the APOE gene is the best genetic hazard component for sporadic, late onset AD, and is additionally related with chance for kind two diabetes mellitus (T2DM). Neuropathological research mentioned the+ very best wide variety of AD lesions in Genius tissue of e4 diabetic patients. However different research assessing AD pathology amongst the diabetic populace have produced conflicting reviews and have failed to exhibit an expand in AD-related pathology in diabetic brain. The thiazolidinediones (TZDs), peroxisome proliferator-activated receptor gamma agonists, are peripheral insulin sensitisers used to deal with T2DM. The TZD, pioglitazone, accelerated reminiscence and cognitive features in moderate to average AD patients. Since it is no longer but clear how apoEisoforms have an impact on the development of T2DM and its development to AD, we investigated amyloid beta and tau pathology in APOE knockout mice, carrying human APOEe3 or e4 transgenes after diet-induced insulin resistance with and barring pioglitazone therapy [17].
Simon Simuni, et al. studied that Pioglitazone, an oral hypoglycemic agent, currently failed to exhibit promise as a disease-modifying agent in a 44-week segment two placebo-controlled learn about in 210 Parkinson’s Disorder (PD) subjects. We analysed peripheral biomarkers, which include leukocyte PGC-1α and goal gene expression, plasma interleukin 6 (IL-6) as a marker of inflammation, and urine 8-hydroxydeoxyguanosine (8OHdG) as a marker of oxidative DNA damage. Baseline or modifications from baseline in biomarker ranges have been no longer related with the fee of development of PD. Pioglitazone did not appreciably alter biomarker levels [18].
It was reported in literature by He Feng, et al. that recent evidence has suggested that an essential factor in the pathophysiology of Parkinson's disease is microglia activation. Activated microglia secretes various pro-inflammatory cytokines and neurotoxic mediators, which may contribute to the development of PD while inhibition of microglia activation may have a therapeutic benefit in the treatment of PD. In the present study, using mesencephalic neuron-microglia mixed culture and microglia-enriched culture, it was investigated that rosiglitazone (RGZ), a member of peroxisome proliferator-activated receptor gamma (PPAR-γ) agonists, could inhibit microglia activation. Their results showed that RGZ significantly inhibited lipopolysaccharide (LPS)-induced microglia activation and the production of tumor necrosis factor-alpha (TNF-α), Nitric Oxide (NO), and superoxide. It was further investigated about intracellular signaling systems controlling LPS-activated microglia production of TNF- and NO. Outcomes demonstrated that RGZ prevented p65's phosphorylation and nuclear translocation [19].
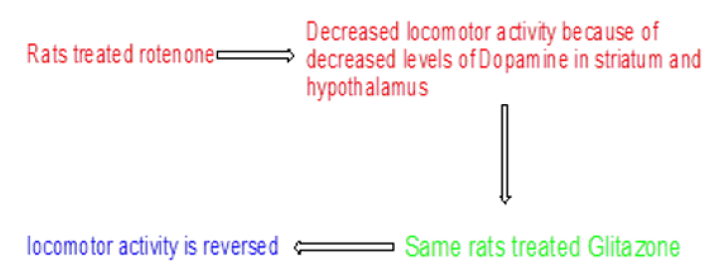
Ulusoy Celik, et al. investigated antioxidant and neuroprotective actions of Pioglitazone and retinoic acid on rotenone induced inflammation in brain of Wistar rats. Rat models were divided into three groups i.e., control, rotenone, and Pioglitazone or Retinoic acid treated rats. Rotenone was administered for 70 days to Wistar rats. At the end of administration of rotenone, Pioglitazone and retinoic acid were administered to the model for 15 days. Rats were evaluated for levels of dopamine in striatum, hippocampus, and hypothalamus. Rotenone affected locomotor activity of rats by reducing dopamine levels in striatum and hippocampus but not in hypothalamus. It was observed that Pioglitazone and Retenoic acid reversed the reduced locomotor activity by increasing the dopamine level in striatum but not in hippocampus. Pioglitazone was found to be more effective in PD than retinoic acid (Figure 8) [20].
It has been reported in the literature by Vural and Seyrek that PPAR-activator with neuroprotective effects through several pathways is pomglitazone (PGT). Both acute and long-term usage is neuroprotective. Chlorpyrifos (CPS), an organophosphate pesticide, is widely documented for its neurotoxic effects on children and can cause cognitive and attention deficit difficulties. The purpose of this work is to look at how PGT protects against CPS neurotoxicity in NB2a cells in the culture medium. The MTT test was used to examine the cell viability and proliferation, and neurite outgrowth was used to analyse the percentage of neurite inhibition. In TUNEL labelling, apoptosis was assessed using the apoptotic index. CPS (25 M) was shown to dramatically limit cell growth, while PGT was able to counteract this concentration-based reduction. CPS (25 M) considerably decreased neurite outgrowth, but PGT (at and above 10 M doses) dramatically restored neurite inhibition. Depending on the concentration, the apoptotic index, which was elevated by CPS (25 M), was seen to decrease by PGT. Organophosphate is bad for human health, and as far as we are aware, there is no cure. PGT may be used to prevent or treat acute toxic effects on neurons in those who have been exposed to chlorpyrifos poisoning [21].
Bonato JM, et al. studies showed that deficits in adult hippocampus neurogenesis have been proposed as a potential pathophysiological cause for the depressed symptoms that Parkinson's disease patients frequently experience (PD). A selective PPAR-agonist known as pioglitazone has been found to have anti-inflammatory and antidepressant properties as well as the ability to influence brain plasticity in several neurodegenerative illnesses. In the current work, a rat model of Parkinson's Disease (PD) that was produced by bilateral 6-hydroxydopamine (6-OHDA) infusions in the substantia nigra pars compact was used to examine the effects of pioglitazone on depressive symptoms and adult hippocampal neurogenesis (SNpc). Rats with SNpc and Ventral Tegmental Area (VTA) neurodegeneration displayed despondent behaviour along with ongoing hippocampus-wide microglial activation. Pioglitazone inhibited microglial activation and decreased mortality in the initial stages of 6-OHDA-inducednigral lesions. In rats with nigral lesions, pioglitazone had antidepressant-like effects and prolonged the survival of neurons in the hippocampus. These findings suggested that pioglitazone exhibits neuroprotective benefits in 6-OHDA-lesioned rats through promoting hippocampus neurogenesis, which may explain some of its antidepressant-like effects [22].
Pioglitazone has a protective effect against behavioural alterations brought by artificially inducing PD in mice, according to by S Divya, et al. Male C57BL6/J mice were given five treatments (25 mg/kg/day i.p.) of the hydrochloride of 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) to induce PD. PGZ (20 mg/kg) was subsequently given orally to the mice for 10 days after MPTP delivery. To evaluate the behavioural deficits associated with PD and the impact of pioglitazone on improving the behavioural manifestations, behavioural studies including open field, rota rod, hang grid, stride length, and olfactory tests were conducted on the eighth day after MPTP injection and the eleventh day after pioglitazone treatment. The results of various behavioural tests conducted in this study revealed that mice treated with MPTP had persistent behavioural deficits in their ability to balance and coordinate their movements on difficult rotarods, hang from a grid, maintain consistency in their stride length while walking, and spontaneously move around in an open field. Intriguingly, prolonged pioglitazone post-treatments led to a considerable improvement in behavioural impairments brought on by MPTP and were successfully reduced [23].
Recent in vitro and animal studies revealed that PPAR agonist medicines, such as diabetes meds like glitazone (GTZ), are neuroprotective in models of Parkinson's disease, according to research by Brauer R, et al. We predicted that patients who received GTZ medications would experience PD at a lower rate than those who received other types of diabetes therapy. We conducted a retrospective cohort study using primary care data from the UK's Clinical Practice Research Datalink (CPRD), matching individuals with diabetes who were newly prescribed GTZ (the GTZ-exposed group) by age, sex, practise, and stage of diabetes treatment with up to five individuals who were prescribed other diabetes medications (another antidiabetic drug-exposed group). From 1999 until the first time a PD diagnosis was entered into the database, the end of the observation period, or the conclusion of the study, patients were monitored. With consideration for potential confounders, an Incidence Rate Ratio (IRR) was computed using conditional Poisson regression. 120,373 additional antidiabetic users were paired with 44,597 GTZ exposed individuals. PD was identified in 175 GTZ exposed people as opposed to 517 in the other group subjected to antidiabetic drugs. In the group that had been exposed to GTZ, the incidence rate of PD was 6.4/10,000 patient per year, whereas it was 8.8/10,000 patient per year in the group that had been given other antidiabetic medications (IRR 0.72, 95% confidence interval 0.60-0.87). Adjustments for possible confounding factors, such as smoking, other drugs, head injury, and the severity of the condition, produced no appreciable effects (fully adjusted IRR 0.75, 0.59-0.94). The risk was decreased in individuals who were now prescribed GTZ (current GTZ-exposed IRR 0.59, 0.46-0.77), but not in those who had previously been prescribed GTZ (past GTZ-exposed IRR 0.85, 0.65-1.10). Our study only included diabetic individuals who were not yet diagnosed with PD when they were first administered GTZ, thus it is impossible to sayif taking GTZ prevents or delays the onset of PD. A current prescription for GTZ is linked to a decrease in the incidence of PD in diabetic individuals. This shows that PPAR-γ pathways might be a useful therapeutic target for Parkinson's disease [24].
According to what Xia P, et al. showed Pioglitazone, a PPAR-agonist, has recently received attention for its ability to slow the course of cerebral ischemia-reperfusion damage. However, there has not been any research on using pioglitazone to treat ischemic stroke via pyroptotic processes. Middle cerebral artery blockage was used to diagnose the brain damage (MCAO). The oxygen-glucose deficit caused primary cultured astrocytes to experience in vitro ischemia (OGD). The levels of PPAR-, pyroptosis-related biomarkers, cytoplasmic translocation of HMGB-1 and RAGE expression, as well as Rac1 activity, were examined using ELISA and Western Blot analysis, respectively. In a rat model of middle cerebral artery blockage, we showed that pioglitazone administered repeatedly intraperitoneally significantly decreased the infarct volume, alleviated neurological impairments, and lowered Rac1 activity (MCAO). Pioglitazone also reduced the expression of HMGB-1 and RAGE in the cytoplasm and the up-regulation of pyroptosis-related indicators in the cerebral penumbra cortex. Similar to this, pioglitazone's protective effects on cultured astrocytes were characterised by decreased Rac1 activity, production of proteins linked to pyroptosis, and release of Lactate Dehydrogenase (LDH). However, the application of the PPAR-inhibitor GW9662 rendered these protective benefits of pioglitazone ineffective. Interestingly, OGD-induced pyroptosis of primary cultured astrocytes may be inhibited by Rac1 knockdown in lentivirus using the Rac1 small hair RNA (shRNA). Additionally, the inhibitory effects on pyroptosis caused by OGD can be strengthened by combining Rac1-shRNA with pioglitazone. Pioglitazone's neuroprotection was a result of reduced ischemia/hypoxia-induced pyroptosis and was also linked to PPAR-mediated inhibition of the HGMB-1/RAGE signalling pathway. Furthermore, its function was increased by Rac1 inhibition [25].
According to Xing B, et al. there is growing evidence connecting neuro inflammation to Parkinson's disease. Neuro inflammation is mediated by microglia. Cyclooxygenase 2 and prostaglandin E2 are released following neuronal insults as a result of overactive microglia. In the past, we have demonstrated how the peroxisome proliferator-activated receptor gamma agonist pioglitazone suppresses microglia activation, lowers pro inflammatory markers, and safeguards dopaminergic neurons. Here, we show that pioglitazone protects dopaminergic neurons by reducing excessive microglia activation, preventing nuclear factor kappa-B and jun N-terminal kinase phosphorylation, decreasing the production of cyclooxygenase 2, and lowering prostaglandin E2 generation. Pioglitazone has anti-inflammatory effects, therefore this may help to slow the course of Parkinson's disease [26].
According to Ward A Pedersen, et al. rosiglitazone and other thiazolidinediones boost peripheral insulin sensitivity, and their usage is recommended for treating Alzheimer's disease. Rosiglitazone may have positive benefits in treating Alzheimer's disease, although the exact processes through which this may be the case are yet unknown. In earlier research, we found that when Tg2576 Alzheimer mice are starved overnight, they have much higher blood corticosterone levels than wild-type mice and acquire peripheral insulin resistance with ageing. We also shown that rosiglitazone treatment can improve both flaws. Here, we find that rosiglitazone-treated Tg2576 mice performed better during behavioural testing that involved repeated overnight fasting and had lower serum corticosterone levels than untreated Tg2576 animals. When untreated Tg2576 mice were given metyrapone, a medication that inhibits the synthesis of glucocorticoids, their spatial learning and memory capacities as well as their serum corticosterone levels were comparable to those of mice treated with rosiglitazone. We further note that in the brains of Tg2576 mice, rosiglitazone mitigated decreases in insulin-degrading enzyme (IDE) mRNA and activity and decreased levels of amyloid -peptide (A) 42 without changing amyloid deposition. These findings show that rosiglitazone reduces learning and memory impairments in Tg2576 mice, and they also imply that the drug's effects on learning and memory, brain IDE levels, and brain A42 levels in the animals may be attributed to its glucocorticoid-lowering effects [27].
Recent developments in clinical, pathology, and neurological investigations have found disease-modifying treatment options for Alzheimer's disease that are now in clinical trials, according to Emma LA, et al. This has brought to light the requirement for trustworthy and practical biomarkers for early illness detection and a quick indication of medication success. It was discussed that identification and evaluation of several potential biomarkers in Alzheimer's patients and the relationship between those biomarkers and the therapeutic effectiveness of rosiglitazone as measured by a change in the Alzheimer's Disease Assessment Scale-Cognitive (ADAS-Cog). Open platform proteomics was used to analyse the plasma of 41 Alzheimer's patients both before and after treatment with 8 mg of rosiglitazone for 24 weeks. Following treatment with rosiglitazone, a comparison of protein expression revealed 97 proteins to be differently expressed with a p-value of less than 0.01. In order to confirm the alterations seen, a prioritised list of 10 proteins from this analysis and comparison to recently published results from our lab were examined by immunoassay and/or functional assay in a larger set of samples from the same clinical investigation, showing a rosiglitazone dosage response. At the higher treatment dosages compared to the placebo, a number of these proteins seemed to correlate with change in ADAS-Cog. At the higher dosages, the expression of alpha-2-macroglobulin, complement C1 inhibitor, complement factor H, and apolipoprotein E exhibited a link with ADAS-Cog score (4 mg and 8 mg). In light of the pathology and other newly released data, these results are reviewed [28].
Traumatic Brain Injury (TBI) has been shown by Junchao Y, et al. to be one of the primary causes of death and morbidity in both adults and children throughout the world. Recent research has shown that TBI-induced neuronal cell death and functional loss include both apoptosis and autophagy. Rosiglitazone (RSG), a well-known anti-inflammatory, works by activating PPAR. It is a peroxisome Proliferator-Activated Receptor (PPAR) agonist. RSG may have neuroprotective benefits in animal models of both chronic and acute brain damage, according to earlier research; however, it is yet unclear if RSG contributes to autophagic neuronal death after TBI. The purpose of the current investigation was to ascertain if RSG exerts its neuroprotective effects by reducing neuronal apoptosis and autophagy after TBI in a rat model. Additionally, the influence of RSG on functional recovery following TBI was assessed. The involvement of RSG in the control of inflammation and glutamate excite toxicity was also examined. The rats were randomly assigned to one of three groups after undergoing controlled cortical impact injury: The sham operation group, the TBI group, and the RSG therapy group. Following TBI, 2 mg/kg of RSG was intra peritoneally administered to the RSG treatment group. According to the findings of the current investigation, RSG therapy after TBI dramatically decreased neuronal apoptosis and autophagy and improved functional recovery. Tumor necrosis factor-alpha and interleukin-6 protein expression levels fell in tandem with these effects. The levels of glutamate transporter-1 protein expression in the cortex of the brain, however, showed no discernible modifications. The current study's findings offer in vivo proof that RSG may have neuroprotective benefits by preventing neuronal apoptosis and autophagy in rats after experimental TBI. The mechanism behind these effects may be related to RSG's anti-inflammatory properties. The current study provides a fresh perspective on the possible application of RSG as a neuroprotective drug in the management of brain injury [29,30].
Sarathlal KC, et al. reported that Alzheimer's Disease (AD) is a neurological condition that poses a danger to one's capacity for thought. A growing body of research indicates that poor insulin signaling and glucose metabolism in the brain are intimately related to Type 2 Diabetic Mellitus (T2DM) and Alzheimer's Disease (AD). Although members of the peroxisome Proliferator-Activated Receptor (PPAR) family, particularly PPAR agonists, are widely known for their ability to increase insulin sensitivity, their clinical use is restricted by their low water solubility, limited brain penetration, and associated toxicity.
Discussion
The purpose of this work is to ascertain whether the streptozotocin (STZ)-induced AD mouse model may benefit from the neuroprotective properties of rosiglitazone embedded nanocarrier technology. By measuring the messenger Ribonulceic acid (mRNA) expression level of genes associated with cognitive function, the in vitro neuroprotective effectiveness of rosiglitazone on SH-SY5Y cells was assessed. STZ (3 mg/kg) was injected Intra Cerebro Ventricularly (ICV) into the lateral ventricles of the mouse brain to cause AD. Both the free form of rosiglitazone and its nano-formulated form were used to treat patients, and both forms allowed for the evaluation of cognitive measures and mRNA expression levels. Rosiglitazone was found to induce neuroprotection in SH-SY5Y cells, as shown by the upregulation of genes related to cognition, including Cyclic-Amp Response Element-Binding Protein (CREB), Brain-Derived Neurotrophic Factor (BDNF), glial cell Derived Neurotrophic Factor (GDNF), and Nerve Growth Factor (NGF). Additionally, the nano-formulated rosiglitazone produced greater neuroprotective efficacy than its unformulated counterpart in animal models of AD as demonstrated by the amelioration of behavioural and cognitive abnormalities, oxido-nitrosative stress, and pro-inflammatory cytokines such as Tumour Necrosis Factor- (TNF-), interleukin-6 (IL-6a), as well as enhanced antioxidant enzymes (Superoxide Dismutase (SOD), Reduced Glutathione (GSH), The findings suggest that rosiglitazone nano formulation exerts potent neuroprotection by upregulating growth factor mRNA expression and decreasing oxidative stress, and that neuro inflammation ultimately shields neurons from damage in AD [31].
Saunders, AM et al. reported that Alzheimer's disease is the prototypical "unmet medical need," responsible for 700,000 deaths in the United States in 2020 and 65% of progressive cognitive impairment among the aged. Caregiving for Alzheimer's patients cost $244 billion in 2019, not considering the emotional and physical toll on caretakers. Despite this gloomy fact, there are currently no medications that can lower the likelihood of getting AD or provide long-term mitigation of its most damaging symptoms. In this review, we highlight important elements of Alzheimer's disease biology and genetics and discuss how pioglitazone improves numerous pathophysiological factors of AD. Animal studies indicate that pioglitazone can be both corrective and protective, and that its effectiveness is increased in a time- and dose-dependent way. However, the dose-effect relations are neither monotonic nor sigmoid. Small-scale, unblinded pilot trials appear to support longitudinal cohort studies' findings that it slows the onset of dementia in those with pre-existing type 2 diabetes mellitus. Clinical studies that were placebo-controlled and blinded, however, did not support this conclusion; we therefore, address potential reasons for these disparities [32].
Rahman SO, et al. Complications of the Alzheimer’s ailment (AD) have made the improvement of its therapeutic intervention pretty a difficult task. Numerous researches have supported the hypothesis that central insulin resistance performs a vast position in AD. Serine phosphorylation of Insulin Receptor Substrate-1 (IRS-1) has been discovered to be a contributing element in neuronal insulin resistance. Pioglitazone (PIO) is a peroxisome proliferator-activated receptor gamma (PPAR) activator, regarded for its great anti-diabetic functions, has additionally confirmed neuroprotective action [33].
In a study by Risner, et al., mild-to-moderate AD patients were randomised to receive a placebo or 2, 4, or 8 mg of the medication rosiglitazone (RSG). The AD Assessment Scale-Cognitive (ADAS-Cog) mean change from baseline and the Clinician's Interview-Based Impression of Change plus were the primary endpoints at Week 24. Caregiver Results were also stratified by apolipoprotein E (APOE) genotype (n-323) and input global scores in the intention-to-treat group (N-511). No RSG dosage or placebo showed any statistically meaningful differences on the key end points. APOE 4 allele status and ADAS-Cog had a significant interaction (P-0.014). Exploratory analysis showed a substantial improvement in ADAS-Cog in individuals with APOE 4 negative status on 8 mg RSG (P-0.024; not multiplicity adjusted). Patients who tested positive for APOE 4 did not improve and instead exhibited a decrease at the lowest RSG dosage (P-0.012; multiplicity unadjusted). Exploratory investigations indicated that, in response to RSG, APOE 4 non-carriers showed improvements in cognitive and functional abilities, but APOE 4 allele carriers showed no improvements and some decrease was seen. These early results need to be verified in pertinent clinical investigations [34].
Papadopoulos P, et al., documented the effects of the drug pioglitazone on a mouse model of Alzheimer's disease with both amyloid-beta and cerebrovascular pathology. The study found that pioglitazone improved reversal learning and reduced neuro inflammation in the mice, but did not improve spatial learning and memory deficits and even exacerbated cerebrovascular deficits. The study suggests that pioglitazone may be beneficial in managing certain aspects of Alzheimer's disease but may not be effective in treating cerebrovascular pathology. Pioglitazone countered cortical neuroinflammation, neurometabolic and neurovascular coupling responses to increased neuronal activity in sensory pathways, with an age-dependent improvement in reversal learning, pointing to benefits at several levels of the AD pathology and to potential use as a combined therapy. However, the study also found that chronic pioglitazone therapy may have a deleterious effect on dilatory function, particularly those involving NO signaling, which may be relevant to AD patients with comorbid cardiovascular diseases. Overall, the study's findings suggest that pioglitazone may be a promising treatment for AD, but further research is needed to fully understand its potential benefits and risks [35].
Prakash A, et al. investigated the effects of pioglitazone on cognitive impairment in an animal model of Alzheimer's disease induced by beta-amyloid. The study found that pioglitazone may have therapeutic potential in Alzheimer's disease. Beta-amyloid is believed to be a central player in the pathogenesis of Alzheimer's disease. The amyloidogenesis hypothesis has been the prevailing central pathogenic event in AD. Most drug discovery efforts have been concentrated on reducing/modulating beta-amyloid production. The beta-amyloid aggregates located around neurons not only have a direct toxic effect on the neurons but also enhance the susceptibility of neuronal cells to free radicals and other harmful factors, leading to widespread neurodegeneration and neuro inflammation [36].
Chen J, et al., proved the potential therapeutic effects of the anti-diabetes drug pioglitazone on Alzheimer's Disease (AD). The study found that pioglitazone inhibited the activity of cyclin-dependent kinase 5 (Cdk5), which is believed to be associated with the pathogenesis of AD. Inhibition of Cdk5 activity reversed dendritic spine loss caused by amyloid-β oligomers, a hallmark of AD. Pioglitazone treatment also improved long-term potentiation and spatial memory in AD mouse models. These findings suggest that pioglitazone may have potential as a therapeutic drug for AD. Cdk5 is involved in the regulation of synaptic plasticity and deregulation of its kinase activity contributes to synaptic loss and dysfunction, resulting in neuronal network impairment and cognitive decline in Alzheimer's disease. Cdk5 activity is robustly upregulated in postmortem Alzheimer's disease brains. Inhibition of Cdk5 activity can prevent neuronal loss and exert some protective effect in mouse models of Alzheimer's disease. Therefore, inhibition of Cdk5 activity can be a promising therapeutic strategy for Alzheimer's disease intervention. Pioglitazone treatment improves long-term potentiation and spatial memory in AD mouse models by inhibiting the hyper-activation of Cdk5 kinase activity. Pioglitazone decreases the p35 protein level, which is a co-activator of Cdk5, and thus reduces Cdk5 activity [37].
Chang KL, et al. investigated the influence of drug transporters and stereoselectivity on the brain penetration of pioglitazone, a potential medicine for Alzheimer's disease. The researchers found that inhibiting P-glycoprotein increased the brain penetration of pioglitazone, while inhibiting breast cancer resistance protein had less effect. They also discovered that (+)-pioglitazone had higher brain exposure levels compared to (-)-pioglitazone. These findings suggest that P-gp may act as a barrier to prevent pioglitazone from entering the brain, and dosing with (1)-pioglitazone may improve the development of pioglitazone treatment for Alzheimer's disease. P-glycoprotein (P-gp) is a drug efflux transporter present at the Blood-Brain Barrier (BBB) that limits the brain penetration of pioglitazone, a potential medicine against Alzheimer's disease. Inhibition of P-gp significantly increases brain penetration of pioglitazone, indicating that P-gp acts as a stereoselective barrier to prevent pioglitazone entry into the brain [38].
There is a study performed by Barbiero J. K. et al. which revealed the role of PPAR receptoragonists in Parkinsonism. Pioglitazone and Fenofibrate were injected in rat models of PD induced by intranigral 1-methyl-4-phenyl-1,2,3,6-tetrahyropyridine (MPTP). This research proved that PPAR agonist Pioglitazone protected against depressive like behaviour and hypolocomotion, showed cognitive improvements and protected from domanergic neurodegeneration caused by MPTP. Both drugs, Pioglitazone and fenofibrate, showed protection against caspase-3, an enzyme in apoptosis steps responsible for pathology of PD [39].
Fernandez-Martos CM, et al. investigated the effects of combining the drugs leptin and pioglitazone in a mouse model of Alzheimer's disease. The study found that the combination treatment led to improvements in spatial memory, a decrease in brain amyloid levels, and improvements in pathological changes associated with the disease. It was found that the effect of combination of leptin and pioglitazone treatment for 6 months old APP/PS1 transgenic AD mouse model was significant in terms of reduction in saptail memory deficits and brain β-amyloid levels. This combination of drugs also reduced plaque associated damage to neurons and synapses. It was suggested that combining leptin and pioglitazone can be a better approach for Alzheimer's disease [40].
Chang KL, et al. investigated the role of pioglitazone in metabolic dysfunction in the cortex and cerebellum of mice with Alzheimer's disease. The researchers utilized metabolomics (metabolites used for study their interaction with biological system) to determine metabolic conditions of different part of the brain. It was observed that mice treated with pioglitazone lowered amyloid beta levels, increased antioxidant capacity in cortex and cerebellum parts. The investigators used GC-TOF-MS-based metabolic profiling analysis to determine the metabolic conditions of different parts of the brain of APP/PS1 transgenic mice [41].
Shetty AB, et al. studied novel derivatives of glitazone for the treatment of neurodegenerative disorders. The derivatives were selectively determined for their ability to reverse rotenone-induced neurotoxicity in mouse model. The researchers observed the significant activity of glitazone derivatives for neuroprotection. The methods exploited for studies are molecular docking, acute toxicity testing, pharmacokinetic studies, and evaluation of neuroprotective ability. Neuroprotection activity was justified by prevention against oxidative stress induced by rotenone. Toxicity studies showed the novel glitazone derivatives are safe to use. The testing dose was 300 mg/kg [42].
Shengbo J, et al. investigated the effects of pioglitazone, a drug that activates a specific receptor in the brain, on short- and long-term outcomes after mild brain ischemia. The researchers discovered that pioglitazone had a positive impact in the short term by reducing the size of brain lesions and the number of microglia in the ischemic area. However, it did not show any effect on long-term outcomes such as motor function and learning and memory. The study emphasizes the importance of considering longer-term outcomes in stroke research. 129/SV mice were used as model and were subjected to filamentous middle artery occlusion for 30 min followed by reperfusion. After this, the animal models were regularly treated with pioglitazone (20 mg/kg) through intraperitoneal route [43].
Pizcueta P, et al. stated that Leriglitazone, a Blood-Brain Barrier (BBB) penetrant PPARγ agonist, has shown potential as a therapeutic intervention for neuroinflammatory and neurodegenerative diseases in the central nervous system (CNS). PPARγ activation can exert anti-inflammatory effects and promote tissue repair, making it an attractive target for intervention. Preclinical models have demonstrated the efficacy of leriglitazone in diseases such as multiple sclerosis and cerebral adrenoleukodystrophy, where it can protect oligodendrocytes and promote remyelination. Furthermore, leriglitazone is involved in the regulation of mitochondrial function and biogenesis, crucial for energy metabolism in the CNS. While thiazolidinediones (TZDs), such as pioglitazone, are PPAR-γ agonists primarily used to treat type 2 diabetes mellitus, they have also shown potential in the treatment of neurodegenerative diseases like Parkinson's disease. However, TZDs carry off-target effects and adverse reactions, including fluid retention, weight gain, bone loss, and congestive heart failure. Though promising in preclinical studies, the translation of PPAR-γ agonists into successful clinical applications for CNS diseases has been limited. Positive effects have been observed in small cohorts of patients with Alzheimer's disease and young individuals with autism [44].
This article delves into the intricate relationship between Type 2 Diabetes (T2D) and dementia, exploring the potential therapeutic avenues offered by antidiabetic medications. The heightened risk of dementia among T2D patients, attributed to hyperglycaemia-induced amyloid beta plaque formation and insulin resistance-driven oxidative stress, is underscored. Notably, Thiazolidinediones, such as Pioglitazone, emerge as promising agents, demonstrating a substantial 22% reduced dementia risk compared to Metformin. The multifaceted benefits of Glitazones, including improved glycaemic control and lipid profile modulation, are evident, particularly in extended usage among elderly and obese individuals. The prospect of repurposing diabetes medications for dementia treatment is emphasized, hinging on a comprehensive understanding of their pharmacological attributes and potential impact on central glucose metabolism. As we navigate this evolving landscape, the convergence of pharmacological insights and clinical studies holds the potential to unlock innovative dementia interventions, offering a beacon of hope for a dementia-free future [45].
Conclusion
Glitazone is a vital part of heterocyclic compounds with multifarious biological activities. Most of the glitazone derivatives show neuroprotective activity by activating PPAR-γ receptors. Some studies also showed that anti-inflammatory activity of glitazone is not only via PPAR-γ receptor. Also glitazone inhibits secretion of neuro inflammatory agents cytokine and chemokine from astrocytes. Repeated intraperitoneal administration of pioglitazone appreciably decreased the infarct volume, expanded neurological deficits which prevents the chances of neurodegeneration by ischaemia. These effects validate PPAR-γ as a goal to forestall neurodegeneration in Alzheimer’s and other neurodegenerative disorders. Glitazones also found to reduce the inflammation by suppressing COX-2 enzyme and lowering the production of PGE-2. Glitazones also lower the activity of Rac-1.
Conflict of Interest
There is no conflict of interest.
References
- Breijyeh Z, et al. Molecules. 2020;25(24):5789.
[Crossref] [Google Scholar] [PubMed]
- Fan L, et al. Front Neurol. 2020;10:1312.
[Crossref] [Google Scholar] [PubMed]
- Zhang WY, et al. Arterioscler Thromb Vasc Biol. 2008;28(12):2312-2318.
[Crossref] [Google Scholar] [PubMed]
- Qiu D, et al. Int J Clin Pharmacol Ther. 2015;53(9):746-752.
[Crossref] [Google Scholar] [PubMed]
- Wu CH, et al. Acta Cardiol Sin. 2009;25:127-133.
- Martin HL, et al. Exp Neurol. 2012;235(2):528-538.
[Crossref] [Google Scholar] [PubMed]
- Xu S, et al. Neurosci Lett. 2014;578:7-11.
[Crossref] [Google Scholar] [PubMed]
- Machado MM, et al. Pharmacol Rep. 2019;71(4):556-564.
[Crossref] [Google Scholar] [PubMed]
- Justin A, et al. Neurotox Res. 2020;37:508-524.
[Crossref] [Google Scholar] [PubMed]
- Blackburn JK, et al. Neurochem Int. 2022;152:105222.
[Crossref] [Google Scholar] [PubMed]
- Hassanzadeh K, et al. Brain Res Bull. 2021;173:184-192.
[Crossref] [Google Scholar] [PubMed]
- Rodríguez-Pascau L, et al. Sci Transl Med. 2021;13(596):eabc0555.
[Crossref] [Google Scholar] [PubMed]
- Justin A, et al. Front Neurosci. 2020;14:530148.
[Crossref] [Google Scholar] [PubMed]
- Wang Y, et al. PPAR Res. 2017;2017:4089214.
[Crossref] [Google Scholar] [PubMed]
- Thal SC, et al. J Neurotrauma. 2011;28(6):983-993.
[Crossref] [Google Scholar] [PubMed]
- Swanson CR, et al. J Neuroinflammation. 2011;8:1-4.
[Crossref] [Google Scholar] [PubMed]
- To AW, et al. PloS One. 2011;6(2):e16991.
[Crossref] [Google Scholar] [PubMed]
- Neurol L. Lancet Neurol. 2015;14(8):795-803.
- Yu H, et al. Aging Dis. 2022;13(6):1591.
[Crossref] [Google Scholar] [PubMed]
- Ulusoy GK, et al. Brain Res Bull. 2011;85(6):380-384.
[Crossref] [Google Scholar] [PubMed]
- Vural K, et al. Kafkas Üniv Vet Fak Derg. 2019;25(1):1-8.
- Bonato JM, et al. Exp Neurol. 2018;300:188-200.
[Crossref] [Google Scholar] [PubMed]
- Subashree S, et al. Biolife. 2014;2(3):890–899.
- Brauer R, et al. PLoS Med. 2015;12(7):e1001854.
[Crossref] [Google Scholar] [PubMed]
- Xia P, et al. Cell Physiol Biochem. 2018;45(6):2351-2368.
[Crossref] [Google Scholar] [PubMed]
- Xing B, et al. J Neuroimmunol. 2007;192(1-2):89-98.
[Crossref] [Google Scholar] [PubMed]
- Pedersen WA, et al. Exp Neurol. 2006;199(2):265-273.
[Crossref] [Google Scholar] [PubMed]
- Akuffo EL, et al. Biomarkers. 2008;13(6):618-636.
[Crossref] [Google Scholar] [PubMed]
- Harrington C, et al. Curr Alzheimer Res. 2011;8(5):592-606.
[Crossref] [Google Scholar] [PubMed]
- Yao J, et al. Mol Med Rep. 2015;12(5):6591-6597.
[Crossref] [Google Scholar] [PubMed]
- Kakoty V, et al. Neurosci Lett. 2021;750:135769.
[Crossref] [Google Scholar] [PubMed]
- Saunders AM, et al. Front Neurosci. 2021;15:666958.
[Crossref] [Google Scholar] [PubMed]
- Rahman SO, et al. Biomed Pharmacother. 2019;110:47-58.
[Crossref] [Google Scholar] [PubMed]
- Risner ME, et al. Pharmacogenomics J. 2006;6(4):246-254.
[Crossref] [Google Scholar] [PubMed]
- Papadopoulos P, et al. PloS One. 2013;8(7):e68612.
[Crossref] [Google Scholar] [PubMed]
- Prakash A, et al. Neurotox Res. 2014;25:335-347.
[Crossref] [Google Scholar] [PubMed]
- Chen J, et al. Plos One. 2015;10(4):e0123864.
[Crossref] [Google Scholar] [PubMed]
- Chang KL, et al. Sci Rep. 2015;5(1):9000.
[Crossref] [Google Scholar] [PubMed]
- Barbiero JK, et al. Behav Brain Res. 2014;274:390-399.
[Crossref] [Google Scholar] [PubMed]
- Fernandez-Martos CM, et al. Alzheimers Dement (N Y). 2017;3(1):92-106.
[Crossref] [Google Scholar] [PubMed]
- Chang KL, et al. Mol Neurobiol. 2019;56(11):7267-7283.
[Crossref] [Google Scholar] [PubMed]
- Shetty AB, et al. J App Pharma Sci. 2021;11(10):42-49.
- Ji S, et al. Exp Neurol. 2009;216(2):321-328.
[Crossref] [Google Scholar] [PubMed]
- Pizcueta P, et al. Int J Mol Sci. 2023;24(4):3201.
[Crossref] [Google Scholar] [PubMed]
- Fareed A, et al. Ann Med Surg. 2023;85(4):1296-1297.
[Crossref] [Google Scholar] [PubMed]