Original Articles: 2025 Vol: 17 Issue: 1
Analytical Method Development and Validation for Simultaneous Estimation of Valsartan and Lisinopril in Bulk and Tablet Dosages Form Using HPLC
Sunil Kumar Chaudhary1, Parul Bisht2*
Department of Chemistry, Lumbini Buddhist University, Lumbini, Nepal
Department of Chemistry, Uttarakhand Sanskrit University, Uttarakhand, India
- Corresponding Author:
- Sunil Kumar Chaudhary
Department of Chemistry
Lumbini Buddhist University,
Lumbini,
Nepal
Received: 12-Jun-2024, Manuscript No. JOCPR-24-138777; Editor assigned: 14-Jun-2024, PreQC No. JOCPR-24-138777 (PQ); Reviewed: 28-Jun-2024, QC No. JOCPR-24-138777; Revised: 26-Dec-2024, Manuscript No. JOCPR-24-138777 (R); Published: 02-Jan-2025, DOI:10.37532/0975-7384.2025.17(01).230.
Citation: Chaudhary SK, et al. 2025. Analytical Method Development and Validation for Simultaneous Estimation of Valsartan and Lisinopril in Bulk and Tablet Dosages Form Using HPLC. J. Chem Pharm. Res., 17:230.
Copyright: © 2025 Chaudhary SK, et al. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Abstract
Objective: The purpose of gift examines became to increase an analytical method and validation for the simultaneous estimation of valsartan and lisinopril in bulk a pill dosage form and to make sure the pleasant precision and accuracy via the use of HPLC as well as to increase a manner that can be carried out for the habitual evaluation in laboratories and in-house approach for analysis.
Introduction: Analytical approach validation is the method of demonstrating that the analytical strategies are suitable for their intended use. according to FDA tenet, analytic technique validation is an issue of setting up documented proof that gives an excessive diploma of guarantee that the desired approach will always offer accurate check consequences that evaluate a product in opposition to its described specification and nice attributes. Analytic methods are intended to set up the identity, purity, bodily characteristics and potency of the medicine that we use. Analytical strategies are required to represent drug substance and drug product composition during all phases of pharmaceutical improvement.
Materials and Methods: Analytical method improvement and validation were performed by using the usage of reverse section HPLC. Separation of the drug was achieved on a opposite phase C8 column using a cell segment including phosphate buffer and methanol in the ratio of 35:65 v/v. The drift rate become 0.8 mL/min and the detection wavelength became 237 nm. Usual analytical performance characteristics that were considered inside the validation of the kind of approach are specificity, linearity, accuracy, precision, LoD, LoQ, robustness and gadget suitability.
Results: The peaks were discovered by way of a UV-detector at 237 nm and the retention time for each the medication changed into 2.664 and 3.423 min. the percentage restoration became 99-100% for both the medication. Technique validation was executed for all above new methods evolved. Linearity parameter for the method became analysed with the aid of making ready answer of 5 stages of concentrations. Calibration curve for concentration versus height regions changed into located linear. Precision changed into studied with the aid of getting ready solutions in six replicates and analyzed, % RSD become calculated for the peak regions of every drug. Accuracy for new techniques advanced, evaluated through using the standard solutions of three specific concentrations (50%, 100% and 150%) into sample answer and their percentage recovery at each stage become decided. Robustness for above methods became completed at exclusive go with the flow prices and at one-of-a-kind organic composition, preferred and sample answers have been organized and analyzed at +0.1 ml/min and at +10% and advanced method located to be solid on planned variation.
Conclusion: Parameters evaluated for validating novel RP-HPLC strategies for improvement of analytical method and validation for the simultaneous determination of above dosage paperwork meet the necessities of ICH pointers. The developed techniques are easy, particular, cost effective and fast. These newly
Keywords
Valsartan, Lisinopril, RP-HPLC, Method development, Validation
Introduction
In drug development, analytical method performs complete roles which can be essential of quality of medication. It assures that a drug product meets the mounted fashionable, is solid and could preserve to fulfill purported high-quality throughout its shelf life [1].
Pharmaceutical analysis performs an essential position right from the checking out of raw materials; in-procedure excellent exams and analysis of finished products [2]. Pharmaceutical analysis is considered to determine the identification, electricity, excellent and purity of drug samples [3].
In analytical chemistry, it's far of top importance to benefit statistics about the qualitative and quantitative compositions of substances and chemical species, that is, to find out what a substance is composed of and exactly how lots [4]. In general terms, pharmaceutical analysis incorporates of those approaches important to determine the "identification, power, nice and purity" of medication [5].
Analytical strategies are required to symbolize the drug materials and drug product composition at some point of all phases of pharmaceutical development [6]. Early segment strategies must aid changes in synthetic routes and dosage shape and elucidate the structures and levels of impurities [7]. In later levels, dreams change to the development of fast and robust methods for launch and balance evaluation [8].
Analysis includes a extensive range of simple and instrumental analytical methods, but the most widely used methods for exceptional guarantee are spectroscopy and chromatography [9]. most quantitative evaluation requires measuring specified components within the presence of pattern matrix and/or related materials; consequently, isolation or separation of the additives are required previous such evaluation [10]. In such cases chromatographic strategies are used for quantitative evaluation. In case, in which matrix interference is not discovered quantitative measurements are made using spectroscopic or titration methods [11].
Method validation is a crucial a part of approach development [12]. It's miles the procedure of demonstrating that analytical strategies are appropriate for their intended use and that they guide the identity, great, purity, and potency of the drug materials and drug products [13]. Genuinely, method validation is the process of proving that an analytical technique is appropriate for its meant reason [14].
High performance liquid chromatography
High stress liquid chromatography was evolved within the mid-1970 and quick progressed with the development of column packing substances and the extra comfort of on line detectors [15]. Inside the past due 1970's, new strategies which include reverse section liquid chromatography allowed for advanced separation between very comparable compounds via the 1980's HPLC became usually used for the separation of chemical compounds [16]. Computers and automation delivered to the benefit of HPLC [17]. Liquid Chromatography (LC) is a bodily separation technique conducted in the liquid segment [18]. Analyst is compelled to waft through a column under excessive pressure. Then it is separated into its constituent components via distributing among the cell segment (a flowing liquid) and a stationary section (sorbents packed internal a column) [19].
Four main separation modes of HPLC are everyday phase, reversed phase, ion alternate chromatography, and size exclusion chromatography (gel permeation and gel filtration chromatography [20].
Normal-Phase Chromatography (NPC)
NPC is the traditional separation mode based on adsorption/desorption of the analyst onto a polar stationary segment (commonly silica or alumina). On this method, non-polar compounds journey faster and are eluted first because of the decrease affinity between the non-polar compounds and the desk bound segment. Polar compounds are retained for an extended time due to their better affinity in the direction of the stationary section. Regular phase mode of separation is, consequently, no longer normally used for pharmaceutical applications due to the fact most of the drug molecules are polar in nature and for this reason take longer time to elute.
Reversed-Phase Chromatography (RPC)
Reversed section mode is the most well-known mode for analytical and preparative separations of compounds of hobby in chemical, organic, pharmaceutical, food and biomedical sciences. On this mode, the table certain section is non-polar hydrophobic packing with octyl or octadecyl sensible institution bonded to silica gel and the mobile section is a polar solvent. An aqueous mobile segment lets in the use of secondary solute chemical equilibrium (which includes ionization manipulate, ion suppression, ion pairing and complexation) to govern retention and selectivity. The polar compound gets eluted first on this mode and nonpolar compounds are retained for longer time. As maximum of the medicine and prescription drugs are polar in nature, they are now not retained for longer instances and therefore elute quicker. The particular columns used are Octadecylsilane (ODS) or C8, C4 and so forth., (within the order of increasing polarity of the desk certain segment).
Columns are the coronary heart of HPLC. Liquid chromatographic columns are generally produced from clean bore 2b4ddebc610f0ebc488d9c02eb20a2e5 tubing. Sometimes made from heavy walled glass tubings and polymer tubings which include PEEK. Shield columns are brought earlier than analytical columns to increase the life of analytical columns, thru putting off now not nice particulate depend and contaminants from solvents however additionally pattern additives that bind irreversibly to the table certain phase. Analytical columns tiers from 5-25 cm prolonged; internal diameter is frequently 3-5 mm; the most not unusual particle period of packing is 3-5 μm.
Number one varieties of column packing utilized in LC are pellicular and porous debris. In pellicular packing, spherical, nonporous, glass or polymer beads are used. A skinny layer of silica, alumina, polystyrene-divinylbenzene artificial resin, or an ion-trade resin changed into deposited at the ground of those beads. In the normal porous particle packing of LC includes silica, alumina, polystyrene-divinylbenzene synthetic resin, or an ion-trade resin. Columns for the bonded segment chromatography is ready with the resource of floor functionalization of silica. The ground of fully hydrolysed silica is made of chemically reactive silanol companies. The most useful bonded segment coatings are siloxanes shaped via manner of the reaction of the hydrolyzed floor with organochlorosilanes.
Method development and design of separation method
Strategies for analysing capsules in unmarried or multi issue dosage paperwork may be advanced, furnished one has understanding approximately the character of the pattern, namely, its molecular weight, polarity, ionic man or woman and the solubility parameter. A specific recipe for HPLC, however, can't be supplied because approach improvement entails considerable trial and mistakes techniques. The hardest trouble commonly is wherein to start; what sort of column is worth trying with what sort of cellular phase. In well-known one begins with reversed section chromatography, while the compounds are hydrophilic in nature with many polar organizations and are water soluble. The natural segment concentration required for the cell phase may be estimated with the aid of gradient elution method. For aqueous pattern combos, the first-class manner to begin is with gradient reversed phase chromatography. Gradient may be started out with 5-10% organic segment in the cellular section and the natural segment concentration (methanol or acetonitrile) can be multiplied up to 100% inside 30-45 min. Separation can then be optimized by using changing the preliminary mobile phase composition and the slope of the gradient in step with the chromatogram obtained from the preliminary run. The preliminary cell segment composition can be estimated on the basis of where the compounds of interest were eluted, particularly at what cell section composition. Converting the polarity of cell section can alter elution of drug molecules. The elution power of a mobile segment relies upon its polarity, the more potent the polarity, better is the elution. Ionic samples (acidic or primary) may be separated, if they are found in undissociated form. Dissociation of ionic samples may be suppressed by using the right selection of pH. The pH of the mobile segment must be selected in one of these way that the compounds aren't ionized. If the retention times are too brief, the decrease of the organic segment awareness within the cellular segment may be in steps of 5%. If the retention times are too lengthy, a growth of the organic segment concentration is needed. In UV detection, appropriate analytical effects are received only whilst the wavelength is chosen carefully. This calls for information of the UV spectra of the person present within the sample. If analyte standards are to be had, their UV spectra may be measured previous to HPLC approach development. The molar absorbance at the detection wavelength is also an critical parameter. while peaks aren't detected in the chromatograms, it's miles viable that the sample amount isn't always enough for the detection. An injection of quantity of 20 μL from an answer of one mg/mL concentration usually affords suitable signals for UV active compounds around 220 nm. although the compounds showcase higher λmax, they absorb strongly at decrease wavelength.
It is not always essential to stumble on compounds at their most absorbance. It's miles, however, superb to avoid the detection at the sloppy part of the UV spectrum for particular quantitation. While suited peaks are detected at the chromatogram, the investigation of the peak shapes can help in addition approach development. The addition of peak modifiers to the cellular section can have an effect on the separation of ionic samples. For examples, the retention of the fundamental compounds can be motivated with the aid of the addition of small amounts of triethylamine (a top modifier) to the cellular phase. Similarly, for acidic compounds small quantities of acids which include acetic acid can be used. this could result in useful modifications in selectivity. Whilst tailing or fronting is discovered, it manner that the cell phase isn't always completely compatible with the solutes. In most case the pH isn't always nicely selected and as a result partial dissociation or protonation takes place. While the height shape does not improve by using lower (1-2) or higher (8-9) pH, then ion-pair chromatography may be used. For acidic compounds, cationic ion pair molecules at better pH and for primary compounds, anionic ion-pair molecules at lower pH may be used. For amphoteric solutes or a aggregate of acidic and primary compounds, ion-pair chromatography is the approach of desire. The low solubility of the pattern inside the mobile phase also can reason bad height shapes. It's far continually beneficial to apply the same solvents for the training of pattern solution as the cell section to keep away from precipitation of the compounds inside the column or injector. Optimization may be beginning most effective after an affordable chromatogram has been obtained. An inexpensive chromatogram method that more or much less symmetrical peaks on the chromatogram stumble on all the compounds. With the aid of sight change of the cellular phase composition, the placement of the peaks may be anticipated within the variety of investigated changes. An optimized chromatogram is the only in which all of the peaks are symmetrical and are properly separated in less runtime.
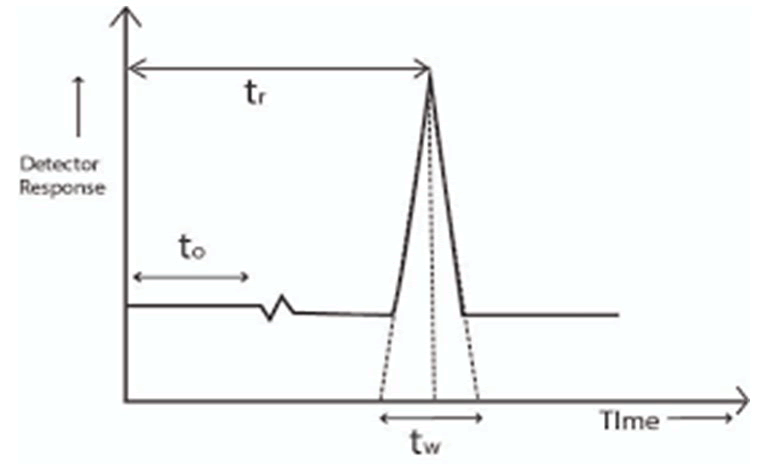
The height decision may be improved through using an extra green column (column with higher theoretical plate number, N) which may be achieved by using a column of smaller particle length, or an extended column. These factors, however, will increase the evaluation time. Go with the flow rate does no longer have an impact on decision, but it has a sturdy effect on the analysis time. Alas, theoretical predictions of mobile section and stationary segment interactions with a given set of sample additives are not usually accurate, but they do help to slim down the picks for technique improvement. The separation scientist must typically carry out a chain of trial and errors experiments with specific cellular segment compositions until a great separation is achived (Figure 1).
Figure 1: HPLC chromatogram.
The parameters that are affected by the changes in chromatographic conditions are:
- Resolution (RS).
- Capacity factor (k").
- Selectivity (α).
- Plate number (N).
- Asymmetry factor (T).
Analytic method development and validation
Analytic technique improvement and validation are non-stop and interconnected sports performed during the drug improvement procedure. Analytical strategies are required to signify drug substance and drug product composition during all phases of pharmaceutical improvement. Early segment methods should guide adjustments in synthetic routes and dosage shape and elucidate the systems and degrees of impurities. In later phases, goals alternate to the improvement of fast and sturdy strategies for launch and stability evaluation that can be transferred to quality devices. Analytic strategies are meant to set up the identity, purity, bodily traits and potency of the drugs that we use.
Analytical method validation is the system of demonstrating that the analytical tactics are appropriate for his or her supposed use. In keeping with FDA guideline, analytic approach validation is a matter of organizing documented proof that offers a high diploma of warranty that the required method will always offer accurate check consequences that examine a product in opposition to its described specification and quality attributes.
The validation process requires quality method development. Whereas validation can be a time-consuming process, methods should not enter the validation phase unless they are fully developed. The relationship of validation and method development can be observed as:
When methods are properly developed, they can be readily validated.
Validation does not make a method better or more efficient.
A validated method does not necessarily imply that it meets all criteria of a properly developed method. Validation acceptance criteria should be based on method development experience.
Method validation is required for the following reasons:
- A new method is being developed.
- Revision of established method.
- When established methods are used in different laboratories and different analysts etc.
- Comparison of methods.
- When quality control indicates method changes.
Advantages of analytical method validation:
- The biggest gain of approach validation is that it builds a degree of confidence, no longer simplest for the developer but also to the person.
- Even though the validation workout may also seem high-priced and time ingesting, it results less expensive, removes frustrating repetitions and ends in higher time control in the end.
- Minor adjustments within the situations together with reagent provider or grade, analytical setup are unavoidable due to obvious reasons however the technique validation absorbs the surprise of such situations and will pay for extra than invested on the procedure.
Guidelines from the following sources provide a framework for performing validation.
- United States Pharmacopoeia (USP)
- International Conference on Harmonization (ICH)
- Food and Drug Administration (FDA)
Validation according to ICH guidelines
Typical validation parameters are:
- Accuracy
- Precision (repeatability, intermediate precision and reproducibility)
- Linearity
- Range
- Specificity
- Robustness
- System suitability testing
- Limit of Detection (LOD) and Limit of Quantitation (LOQ)
Accuracy
Definition: It expresses the closeness of settlement between the price that's familiar either as a conventional actual value or a typical reference price and the cost observed. this is once in a while termed trueness.
The accuracy of an analytical technique is the closeness of check consequences obtained by that method to the genuine cost. The accuracy of the method turned into decided by using recuperation studies. The ICH record on validation method recommends accuracy to be assessed the use of at least 9 determinations over at the very least 3 concentration levels protecting the specified variety. Accuracy need to be said as percentage recovery with the aid of the assay of known added amount of analyte within the pattern or because the difference among the mean and the universal true cost.
Precision
Definition: It expresses the closeness of settlement between a series of measurements acquired from more than one sampling of the equal homogeneous pattern under the prescribed situations.
Precision can be considered at three levels: repeatability, intermediate precision and reproducibility.
Repeatability: It expresses the precision beneath the identical operating situations over a quick c program language period of time. Repeatability is also termed intra-assay precision. Repeatability have to be tested from at least six replications measured at 100% of the check goal attention or from as a minimum nine replications overlaying the complete targeted range.
Intermediate precision: It expresses versions inside laboratories, inclusive of one-of-a-kind days, extraordinary analysts, distinctive device, and so on. The objective of intermediate precision validation is to verify that within the same laboratory the method will provide the same outcomes once the improvement phase is over.
Reproducibility: It expresses the precision between laboratories. The goal of reproducibility is to confirm that the method will offer the equal outcomes in specific laboratories. The reproducibility of an analytical approach is determined with the aid of analyzing aliquots from homogeneous lots in distinct laboratories with extraordinary analysts.
Linearity
Definition: Linearity of an analytical technique is its ability (inside a given variety) to attain take a look at outcomes that are at once proportional to the concentration of analyte inside the pattern.
It may be tested without delay on the drug substance (with the aid of dilution of a general stock solution) and/ separate weighing of artificial combinations of the drug product components, the use of the proposed system.
Range
Definition: Variety of an analytical method is the interval from the top to the lower awareness (quantities) of analyte within the pattern for which it has been tested that the analytical system has an appropriate stage of precision, accuracy and linearity. For the assay of a drug substance or a completed (drug) product: Usually from 80 to one hundred twenty percent of the take a look at attention have to be tested/checked for variety.
Specificity
Definition: It's far the capacity to evaluate unequivocally the analyte inside the presence of additives, which can be anticipated to be gift. Generally, those would possibly encompass impurities, degradants, matrix, and many others.
Robustness
Definition: It is a degree of its potential to remain unaffected with the aid of small, however deliberate variations in technique parameters and gives an indication of its reliability for the duration of normal usage.
System suitability testing
Definition: The exams, primarily based at the idea that the gadget, electronics, analytical operations and samples to be analyzed constitute a fundamental gadget that may be evaluated as such. System suitability test parameters to be hooked up for a particular method depend on the sort of manner being verified. System suitability trying out is a necessary part of approaches.
Limit of detection and limit of quantitation
The detection limit of an individual analytical manner is the lowest amount of analyte in a pattern, which can be detected but not necessarily quantitated as a specific value.
The quantitation limit of a man or woman analytical process is the lowest concentration of analyte in a sample, which can be quantitatively determined with an appropriate level of precision and accuracy.
Numerous techniques for determining are viable, depending on whether or not the manner is a non-instrumental or instrumental.
Based on visual evaluation
Based on signal to noise
Based on the standard deviation of the response and the slope.
The LOD and LOQ were estimated from the set of 5 calibration curves used to determine method linearity. Limit of detection and limit of quantitation can be calculated by the following equation (Figures 2 & 3).
LOD=3.3 (σ/S), LOQ=10 (σ/S)
Where,
σ=Standard deviation of y-intercepts of regression lines
S=Slope of the calibration curve
Drug profile
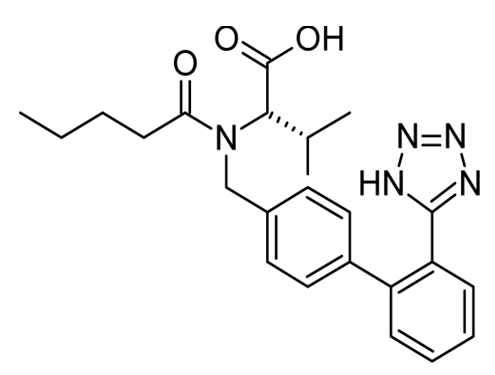
Valsartan
Figure 2: Molecular structure of valsartan.
IUPAC name: (2S)-3-methyl-2-[pentanoyl-[[4-[2-(2H-tetrazol-5-yl) phenyl] phenyl] methyl] amino] butanoic acid.
Molecular formula: C24H29N5O3
Molecular weight: 435.519 g/mol
CAS No: 137862-53-4
Melting point: 116-117°C
Boiling point: 684.9°C
Density: 1.212 ± 0.06 g/cm3 (predicted)
Phase: Powder
Appearance: White to tan
Storage temperature: 2-8°C
Solubility in water: 0.18 g/L soluble in water at 25°C
Solvent solubility: Soluble in ethanol and methanol
BCS classification: II class
Drug category: Anti-hypertensive
Brand names: Diovan, Prexxartan, Valzaar, Valent, Valembic, Starval
Lisinopril
Figure 3: Molecular structure of lisinopril.
IUPAC name: (2S)-1-[(2S)-6-amino-2-[[(1S)-1-carboxy-3-phenylpropyl] amino] hexanoyl] pyrrolidine-2-carboxylic acid
Molecular formula: C21H31N3O5
Molecular weight: 405.5 g/mol
CAS No: 83915-83-7
Melting point: 160°C
Boiling point: 666.4°C at 760 mmHg
Density: 1.212 ± 0.06 g/cm3 (predicted)
Flash point: 356.9ºC
Appearance: White to off white in color.
Storage temperature: 2-8°C
Solubility in water: Soluble in water (approximately 13 mg/L at room temperature)
Solvent solubility: Less soluble in methanol and virtually insoluble in ethanol.
BCS classification: III class
Drug category: Anti-hypertensive
Brand names: Zestril, Prinivil, Zestoretic, Qbrelis
Materials and Methods
Chemicals and reagents
Valsartan and lisinopril reference standard are kindly supply by Nivix Pharmaceutical and Asian Pharmaceutical as a gift sample respectively (Tables 1 and 2).
|
S. NO. |
Name |
Manufacturer |
Grade |
|
1 |
Valsartan |
Sigma-Aldrich |
- |
|
2 |
Lisinopril |
Sigma-Aldrich |
- |
|
3 |
Potasium dihydrogen phosphate |
Merck |
GR |
|
4 |
Sodium perchlorate |
Merck |
GR |
|
5 |
Perchloric acid |
Merck |
GR |
|
6 |
Orthophosphoric acid |
Merck |
GR |
|
7 |
Methanol |
Merck |
HPLC |
|
8 |
Acetonitrile |
Merck |
HPLC |
|
9 |
Water |
Merck |
HPLC |
|
10 |
0.45 µm Nylon filter |
Axivia |
S0761009 |
|
11 |
0.45 µm PVDF filter |
Rankem |
D004A07 |
Table 1: List of chemicals and reagents.
| S. No. | Instrument name | Model |
| 1 | HPLC system | Shimadzu |
| 2 | Analytical balance | Shimadzu |
| 3 | pH meter | Thermo electron corporation orion 2 star |
| 4 | Sonicator | Ultra sonic cleaner power sonic 420 |
| 5 | Vacuum oven | Wadegati |
| 6 | Constant temperature water bath | Thermolab GMP |
Table 2: List of equipment/instruments details.
Analytical method development for the simultaneous estimation of valsartan and lisinopril by RP-HPLC.
Selection of wavelength: 100 μg/ml of valsartan and lisinopril solution were prepared in methanol and thus resulting solution were scanned individually from 190-400 nm in UV-Visible spectrophotometer.
Selection of chromatographic condition: Proper selection of the method depends up on the nature of the sample (ionic/ionisable/neutral molecule), its molecular weight and solubility. The drugs selected in the present study, were polar in nature. Thus reverse phase HPLC was selected for the initial separation because of its simplicity, suitability, ruggedness and its wider usage.
Initial separation condition: Acetonitrile and phosphate buffer were selected as a mobile phase to elute the drug from stationary phase due to its favorable UV transmittance, low viscosity and low back pressure.
Effect of buffer: Because of its better and higher response, Potassium di-hydrogen phosphate were selected for this method.
Effect of pH: The mobile phase pH was optimized using different pH, ranging from 2.0 to 3.0 (pH is adjusted with Ortho phosphoric acid), at a flow rate of 0.5 mL/min. The peak shape and resolution was observed at different pH.
Effect of ionic strength: The phosphate buffer was prepared in different strengths such as 0.01 M, 0.025 M, 0.05 M of potassium di-hydrogen phosphate at pH 2.8. The retention time was decreased by increasing the buffer strength. For the present study, the optimized mobile phase composition phosphate buffer of pH 2.8: Acetonitrile (35:65v/v) was selected, because of the retention times of valsartan and lisinopril were effected due to slight change of ionic strength during analysis.
Preparation of phosphate buffer
7.0 grams of KH2PO4 was weighed into a 1000 ml beaker, dissolved and diluted to 1000 ml with HPLC water. The flask was shaken until the particles get dissolved and volume was made up to the mark with water. The pH was adjusted to 2.8 with ortho phosphoric acid.
Preparation of mobile phase
Mixture of above buffer 350 ml (35%) and 650 ml of acetonitrile HPLC (65%) were mixed and degassed in ultrasonic water bath for 5 minutes and filtered through 0.45 μ filter under vacuum filtration.
Diluents preparation: Mobile phase was used as diluents.
Preparation of standard solution (mixed standard)
A standard solution containing valsartan and lisinopril were prepared by weighing 10 mg of valsartan and 10mg of lisinopril and dissolved in 100 ml mobile phase and the solution was sonicated for 10 min., the volume was made up to the mark with the mobile phase to obtain a stock solution of 100 μg/ml of valsartan and lisinopril.
From the stock solution further dilution were prepared by diluting required volume of mobile phase.
Preparation of sample solution
accurately weighed commercially formulated 10 pills of valsartan and lisinopril and powdered via motor and pistol. The powder equivalent to the quantity of energetic component found in 10 pills turned into transferred into 100 ml volumetric flask, 70 ml of diluents was introduced to it and became shaken by means of mechanical stirrer and sonicated for approximately half-hour by using shaking at intervals of five minutes every and was diluted up to the mark with diluents and allowed to face till the residue settles before taking an aliquot for further dilution (stock solution). the solution became filtered via 45 μm filter before injecting into HPLC machine.
Test procedure
The injection volume 20 μl of blank, standard and sample were injected separately into HPLC system isocratically at a flow rate of 1 ml/min, where the column was maintained at 40°C temperature and the total run time was 8 minutes. The detection was carried out at 252 nm.
Method validation
The objective of validation of an analytical procedure is to demonstrate that it is suitable for its intended purpose. According to ICH Q2B guidelines, typical analytical performance characteristics that should be considered in the validation of the types of methods are:
- Specificity
- Linearity
- Accuracy
- Precision
- Limit of detection
- Limit of quantification
- Robustness
- System suitability
Specificity
Valsartan and lisinopril identification: Check technique were performed via injecting prepared answer of popular and pattern answer into HPLC device where attractiveness standards were chromatogram of general and pattern must be identical with close to retention time.
Establishment of the interference of placebo became conducted in which a pattern of placebo become injected into HPLC gadget as consistent with the take a look at method. Popularity standards are that chromatogram of placebo should not show any peak on the retention time of analyte top consequently, there's no interference because of placebo on the retention time of analyte. Therefore, the approach is specific.
As above interference of clean become hooked up carrying out the test system by means of injecting diluents into the HPLC machine in which chromatogram of clean need to not show any top on the retention time of analyte and there's no interference which prove the method is unique.
Linearity
Accurately weighed 10 mg valsartan and 10 mg lisinopril working standard were transferred into 100 ml volumetric flask and about 70 ml of diluents was added and sonicated to dissolve it completely and the volume was made up to marks with the same solvents.
Linearity was studied by analyzing five standard solutions covering the range of appropriate percentage for valsartan and lisinopril. From the primary stock solution 0.5 ml, 1 ml, 1.5 ml, 2 ml and 2.5 ml of aliquots are pipette into 10 ml volumetric flask and made up to marks with the diluents to give a concentration of 5 μg/ml, 10 μg/ml, 15 μg/ml, 20 μg/ml and 25 μg/ml of valsartan and lisinopril. Each level solution was injected into the HPLC system and the peak area was measured.
Accuracy
The method was determined by performing the assay of valsartan and lisinopril in triplicate for various concentration approximately 50%, 100% and 150% of the working strength of valsartan and lisinopril by injecting into HPLC system as per the test procedure. Amount found and amount added for valsartan and lisinopril, individual recovery and mean recovery values were calculated and average % recovery of valsartan and lisinopril was also calculated.
The acceptance criteria is that the mean % recovery of the valsartan and lisinopril, at each spike level should be not less than 98% and not more than 102%.
Precision
Repeatability: A inventory solution became prepared as required dilution and changed into injected for five instances and the area became measured for all 5 injections in HPLC. The %RSD for the region of 5 mirror injections changed into found to be in the detailed limits.
The reputation criteria are that all individual assays of valsartan and lisinopril capsules ought to be within 98%-102% and relative fashionable deviation of % assay effects must no longer be more than 2.0.
Intermediate precision (analyst to analyst variability): For the intermediate precision also called the ruggedness of the technique precision become achieved on one of a kind day by means of using exclusive column of identical dimensions.
An organized inventory solution become injected via two analysts as according to check approach for 5 instances and the areas of all five injections had been measured in HPLC. The %RSD for the area of five mirror injections become discovered to be within the special limits. The recognition criteria are that each one person assays of valsartan and lisinopril drugs should be inside 98%-102% and relative standard deviation of % assay outcomes need to now not be more than 2% via both the analysts.
Limit of detection
Valsartan
Preparation of 10 μg/ml solution: Accurately weighed 10 mg valsartan working standard were transferred into 100ml volumetric flask and about 70ml of diluents was added and sonicated to dissolve it completely and the volume was made up to marks with the same solvents. Further 1 ml of the above stock solution was pipette into 10 ml volumetric flask and diluted up to the mark with diluents.
Preparation of 0.25% solution at specification level (0.025 μg/ml solution)
In addition, 1 ml of the above inventory answer was pipette into a 10 ml volumetric flask and diluted up to speed with diluents. In addition, 1 ml of the above inventory answer turned into pipette into a 10 ml volumetric flask and diluted up to speed with diluents. 2.5 ml of 0.1 μg/ml solution become pipette in to a 10 ml volumetric flask and diluted on pinnacle of things with the diluents.
Lisinopril
Preparation of 20 μg/ml solution: Accurately weighed 10 mg lisinopril working standard were transferred into 100 ml volumetric flask and about 70 ml of diluents was added and sonicated to dissolve it completely and the volume was made up to marks with the same solvents. Further 2 ml of the above stock solution was pipette into 10 ml volumetric flask and diluted up to the mark with diluents.
Preparation of 0.2% solution at specification level (0.04 μg/ml solution)
Similarly, 1 ml of the above inventory solution modified into pipette into a 10 ml volumetric flask and diluted up to speed with diluents. Similarly, 1 ml of the above stock solution modified into pipette into a 10 ml volumetric flask and diluted up to speed with diluents. 2 ml of 0.2 μg/ml solution became pipette in to a 10 ml volumetric flask and diluted on top of factors with the diluents.
The formula for determination of LOD is
LOD=S/N
Where
S=signal obtained from LOD solution
N=Average baseline noise obtained from blank
Acceptance criteria are that the S/N ratio value should not be more than 3 for LOD solution.
Limit of Quantification (LOQ)
Valsartan
Preparation of 10 μg/ml solution: Accurately weighed 10 mg valsartan working standard were transferred into 100 ml volumetric flask and about 70 ml of diluents was added and sonicated to dissolve it completely and the volume was made up to marks with the same solvents. Further 1 ml of the above stock solution was pipette into 10 ml volumetric flask and diluted up to the mark with diluents.
Preparation of 1% solution at specification level (0.10 μg/ml solution)
Further 1 ml of the above stock answer emerge as pipette into a 10 ml volumetric flask and diluted on top of things with diluents. Yet again 1 ml answer became pipette into a 10 ml volumetric flask and diluted on top of things with diluents.
Lisinopril
Preparation of 20 μg/ml solution
Accurately weighed 10 mg lisinopril working standard were transferred into 100 ml volumetric flask and about 70 ml of diluents was added and sonicated to dissolve it completely and the volume was made up to marks with the same solvents. Further 2 ml of the above stock solution was pipette into 10 ml volumetric flask and diluted up to the mark with diluents.
Preparation of 1.0% solution at specification level (0.2 μg/ml solution)
Further 1 ml of the above inventory answer changed into pipette into a 10 ml volumetric flask and diluted up to the mark with diluents. once more 1 ml answer become pipette into a 10 ml volumetric flask and diluted up to speed with diluents.
LOQ is determined by following formula:
LOQ=S/N
Where
S=Signal obtained from LOD solution (1% of target assay concentration)
N=Average baseline noise obtained from blank
Acceptance criteria are that the S/N ratio value should be 10 for LOD solution.
Robustness
It was determined by way of analysis of aliquots from homogenous masses through differing physical parameters like flow charge and cell section composition, temperature variations which can also range however the responses had been still inside the distinctive limits of the assay.
Effect of variation of flow rate: To decide the impact of variation of go along with the flow charge, it end up numerous at 0.4 ml/min to 0.6 ml/min. general solution of valsartan and lisinopril became prepared and analyzed using the varied go with the flow rates collectively with method go along with the waft price. The results are summarized. On assessment of the above results, it may be concluded that the model in go with the flow charge affected the technique notably. Consequently, it shows that the approach is powerful even with the aid of exchange in the flow price ± 10%. The technique is powerful simplest in a great deal less glide condition. The effect of version of go with the flow charge changed into evaluated.
Recognition standards are that the tailing element for valsartan and lisinopril must no longer be greater than 2.0 in version go with the go with the flow. And the % RSD of asymmetry and retention time for valsartan and lisinopril want to now not be extra than 2.0% for variant in float.
Effect of variation of mobile phase composition: It turned into determine by using converting the ratio of mobile phase in which the natural composition of cellular phase turn out to be severa from 55% to 70%.
Well known answer of valsartan and lisinopril modified into organized and analyzed using the numerous cell segment composition collectively with the actual mobile segment composition within the technique. Sizable solution was organized and injected into the HPLC machine.
Popularity requirements are that the tailing aspect for valsartan and lisinopril must not be greater than 2.0 for model in composition of cell segment. And the % RSD of asymmetry and retention time for valsartan and lisinopril want to no longer be greater than 2.0% for model in composition of cell phase.
System suitability
System suitability was determined by injecting sample solution of valsartan and lisinopril thrice times into HPLC system as per test procedure and its parameter were evaluated from standard chromatograms obtained, by calculating the % RSD of retention time, tailing factor, theoretical plates and peak areas from three replicate injections.
Acceptance criteria
- The %RSD for the retention times of principal peak from 3 replicate injections of each standard solution should be not more than 2.0%
- The number of theoretical plates (N) for the valsartan and lisinopril peaks should be not less than 2000.
- The Tailing factor (T) for the valsartan and lisinopril peaks should be not more than 2.0.
Results and Discussion
Result and discussion of HPLC method
This thesis is aimed to develop the analytical method for simultaneous estimation of valsartan and lisinopril by HPLC method. As there was no single method for the simultaneous estimation of valsartan and lisinopril new analytical method has been developed for the simultaneous estimation of valsartan and lisinopril by HPLC method and validated according to ICH Q2B guideline.
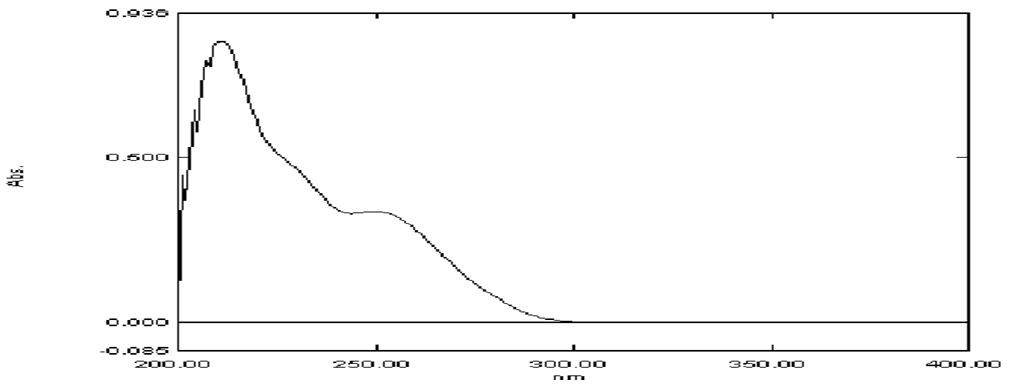
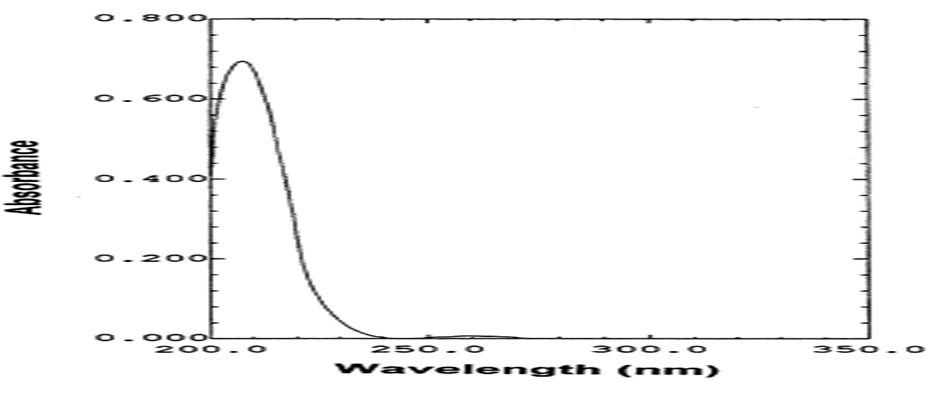
Selection of wavelength
Valsartan and lisinipril solution of 100 μg/ml was prepared separately using methanol as solvent. The solution was scanned individually from 190 to 400 nm in UV-Visible spectrophotometer. The optimal response for the overlain spectrum of valsartan and lisinopril was obtained at 257 nm. Hence the complete method was processed at the wavelength of 237 nm (Figures 4 and 5). Spectrum are shown below.
Figure 4: UV spectrum of Valsartan.
Figure 5: UV spectrum of lisinopril.
Analytical method development
To get good peak resolution, acceptable plate count and tailing factor several trails were made and method was optimized for the simultaneous estimation of valsartan and lisinopril pharmaceutical dosages form (Figures 6,7 and Tables 3,4).
Optimized method
Mobile phase : Phosphate buffer (pH 2.8): Acetonitrile (35:65 v/v)
Diluents : Mobile phase was used as diluents
Chromatographic conditions
Flow rate : 1 min per min
Column : SymmetryXterraC18 (4.6 x 150 mm, 5 mm)
Detector wavelength : 237 nm
Column oven : Ambient
Injection volume : 20 μl
Run time : 10 min
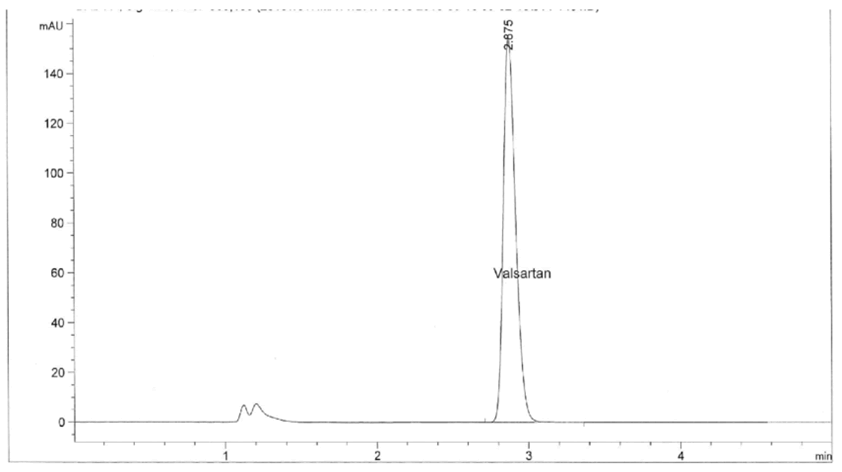
Figure 6: Standard chromatogram of valsartan.
| Name | Retention time | Area | USP tailing | USP plate count |
| Valsartan | 2.71 | 1425053 | 1.04 | 3598 |
Table 3: Standard chromatogram of valsartan.
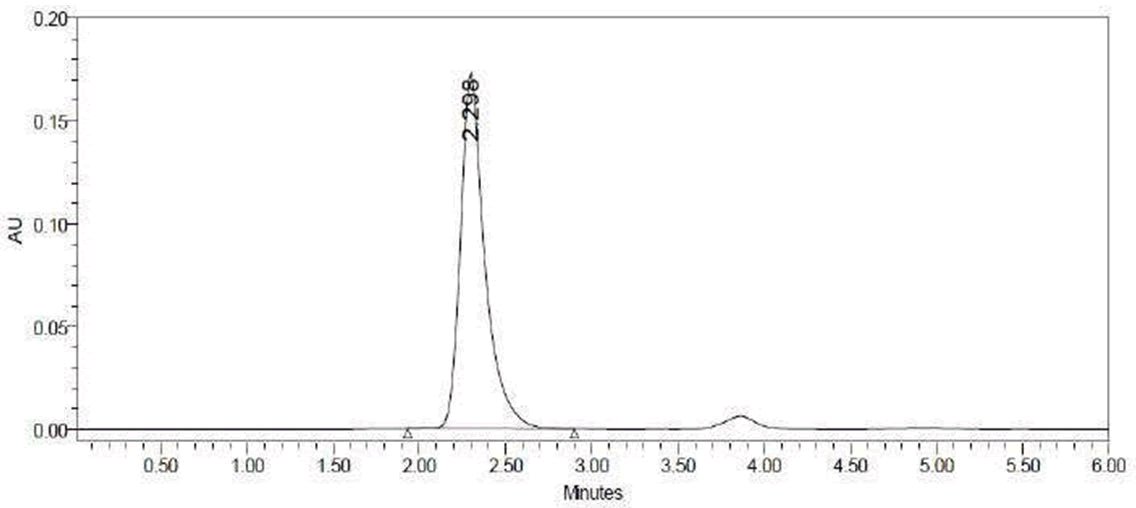
Figure 7: Standard chromatogram of lisinopril.
|
Name |
Retention time |
Area |
USP tailing |
USP plate count |
|
Lisinopril |
2.2 |
1713758 |
1.07 |
4563 |
Table 4: Standard chromatogram of lisinopril.
Specificity
The chromatograms of standard and sample are identical with nearly same retention time. No interference due to placebo and sample at the retention time of analyte which shows that the method was specific. The results were given in the Table 5.
|
S.No |
Sample name |
RT (min) Valsartan |
RT (min) Lisinopril |
|
1 |
Standard |
2.661 |
3.472 |
|
2 |
Sample |
2.663 |
3.469 |
|
3 |
Blank |
- |
- |
|
4 |
Placebo |
- |
- |
Table 5: Specificity data of valsartan and lisinopril.
Result: Chromatograms explain that retention time for standard, sample and formulated product of Valsartan and Lisinopril are same. This proves that, recipients have no effect on the analytical method. On the other hand, blank peak did not overlap drug peak. So the method is highly selective.
Linearity
Calibration curve with concentration verses peak areas was plotted by injecting the prepared solutions (Figures 8 and 9) and the obtained data were subjected to regression analysis using the least squares method and the data was represented in the Tables 6 and 7.
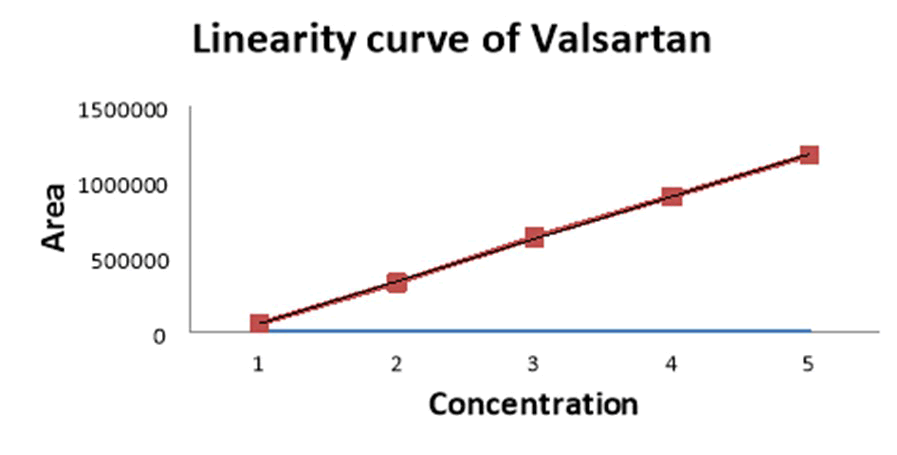
Figure 8: Linearity (calibration) curve of valsartan.
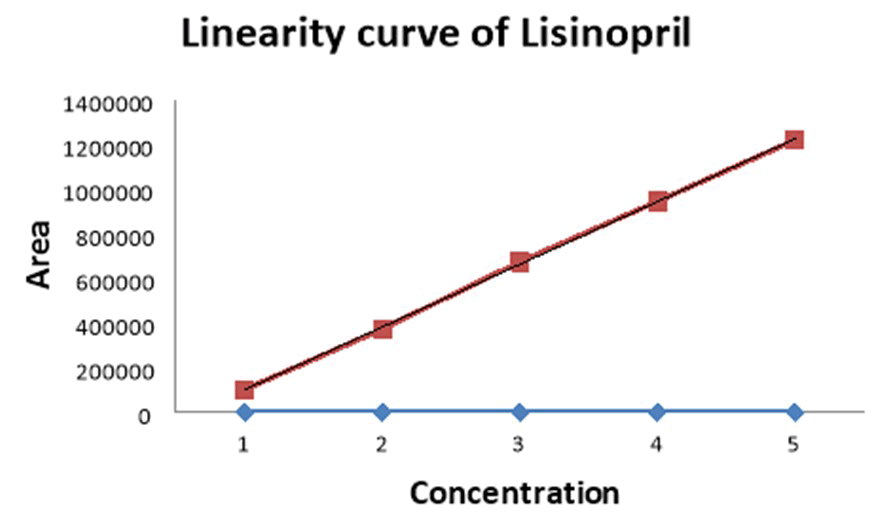
Figure 9: Linearity (calibration) curve of lisinopril.
| S. No | Concentration (µg/ml) | Valsartan |
| Area | ||
| 1 | 5 | 56982 |
| 2 | 10 | 328348 |
| 3 | 15 | 629714 |
| 4 | 20 | 901080 |
| 5 | 25 | 1175446 |
| Correlation coefficient (r²) | 0.999 | |
Table 6: Linearity data of valsartan.
|
S. No |
Concentration (µg/ml) |
Lisinopril |
|
Area |
||
|
1 |
5 |
102880 |
|
2 |
10 |
374246 |
|
3 |
15 |
675612 |
|
4 |
20 |
946978 |
|
5 |
25 |
1221344 |
|
Correlation coefficient (r²) |
0.999 |
|
Table 7: Linearity data of lisinopril.
Result: A linear relationship between peak areas versus concentrations was observed for Valsartan and Lisinopril in the range of 5 μg/ml to 25 μg/ml of nominal concentration. Correlation coefficient was 0.999 and 0.999 for both valsartan and lisinopril which prove that the method is linear in the range of 5 μg/ml to 25 μg/ml (Table 8).
Accuracy
|
Level |
S No. |
% Recovery of valsartan |
Average of valsartan |
% Recovery of lisinopril |
Average of lisinopril |
|
50% |
1 |
99.2 |
99.5 |
99.8 |
99.43 |
|
2 |
99.7 |
99.6 |
|||
|
3 |
99.6 |
98.9 |
|||
|
100% |
1 |
99.4 |
99.63 |
99.6 |
99.73 |
|
2 |
99.9 |
99.4 |
|||
|
3 |
99.6 |
100.2 |
|||
|
150% |
1 |
99.1 |
99.26 |
99.5 |
99.56 |
|
2 |
99.8 |
99.4 |
|||
|
3 |
98.9 |
99.8 |
Table 8: Recovery data of valsartan and lisinopril.
Result: Results of accuracy study are presented in the above table. All the results indicate that the method is highly accurate (Table 9).
Method precision (repeatability)
| S No. | Valsartan | Lisinopril | ||||
| RT (min) | Area | %Assay | RT (min) | Area | %Assay | |
| Injection 1 | 2.664 | 1122498 | 99.6 | 3.439 | 1713758 | 99.4 |
| Injection 2 | 2.665 | 1126986 | 98.2 | 3.45 | 1700467 | 98.99 |
| Injection 3 | 2.666 | 1125272 | 98.6 | 3.444 | 1704762 | 99.2 |
| Injection 4 | 2.662 | 1124239 | 99.5 | 3.472 | 1752656 | 98.98 |
| Injection 5 | 2.666 | 1125567 | 99.4 | 3.336 | 1717583 | 99.54 |
| Injection 6 | 2.665 | 1124392 | 99.7 | 3.401 | 1716273 | 99.89 |
| Mean | 2.664 | 1124826 | 99.16 | 3.423 | 1717583 | 99.33 |
| STDEV | 0 | 1507.83 | 0.61 | 0.04 | 18459.67 | 0.35 |
| % RSD | 0.05 | 0.13 | 0.62 | 1.42 | 1.07 | 0.35 |
Table 9: Summary of RT (min), peak area and % assay for the method precision of valsartan and lisinopril.
Result: Results of variability were summarized in the above Table 9 percentage Relative Standard Deviation (%RSD) was found to be less than 2.0% which proves that method is precise (Tables 10-12).
Limit of detection
| Parameter | Vasartan (µg) | Lisinopril (µg) |
| LOD | 0.06 | 0.04 |
Table 10: LOD value of valsartan and lisinopril.
Limit of quantification
|
Parameter |
Vasartan (µg) |
Lisinopril (µg) |
|
LOQ |
0.01 |
0.803 |
Table 11: LOQ value of valsartan and lisinopril.
Robustness
| Parameter | Normal | Variation | RT (min) | Tailing factor | Theoretical plate | |||
| VAL | LIS | VAL | LIS | VAL | LIS | |||
| Wavelength variation | 237 | 235 | 2.664 | 3.439 | 0.901 | 0.882 | 3589 | 4566 |
| 239 | 2.672 | 3.450 | 0.937 | 0.914 | 3617 | 4974 | ||
| Flow rate variation | 1.0 | 0.8 | 2.238 | 4.280 | 0.962 | 0.891 | 4191 | 4218 |
| 1.2 | 3.310 | 2.986 | 1.07 | 0.894 | 3123 | 4721 | ||
| Column oven temperature variation | 30?C | 25 | 2.698 | 3.427 | 0.899 | 0.906 | 3698 | 4522 |
| 35 | 2.541 | 3.439 | 0.919 | 0.898 | 3924 | 4729 | ||
| Mobile phase variation | 70:30 | 60:40 | 2.632 | 3.898 | 0.927 | 0.991 | 3896 | 4315 |
| 80:20 | 2.498 | 3.412 | 0.968 | 0.996 | 3841 | 4279 | ||
| Where VAL: Valsartan; LIS: Lisinopril | ||||||||
Table 12: Result of robustness of valsartan and lisinopril.
Result: The results of robustness of the present method had shown that changes made in the flow and wavelength did not produce significant changes in analytical results which were presented in the above table. As the changes are not significant, we can say that the method is robust (Tables 13 and 14).
System suitability
| S No. | Sample name | RT (min) | Area | USP plate count | USP tailing factor | ||||
| VAL | LIS | VAL | LIS | VAL | LIS | VAL | LIS | ||
| 1 | Injection 1 | 2.664 | 3.439 | 1122498 | 1713758 | 3598 | 4724 | 0.962 | 0.896 |
| 2 | Injection 2 | 2.665 | 3.441 | 1126986 | 1700467 | 3602 | 4698 | 0.968 | 0.895 |
| 3 | Injection 3 | 2.662 | 3.444 | 1125272 | 1704762 | 3599 | 4692 | 0.969 | 0.896 |
| 4 | Injection 4 | 2.666 | 3.441 | 1124239 | 1752656 | 3611 | 4713 | 0.969 | 0.894 |
| 5 | Injection 5 | 2.665 | 3.438 | 1125567 | 1717583 | 3594 | 4702 | 0.967 | 0.898 |
| 6 | Injection 6 | 2.666 | 3.443 | 1124392 | 1716273 | 3596 | 4696 | 0.963 | 0.896 |
| Mean | 2.66 | 3.424 | 1124826 | 1717583 | 3600 | 4704.16 | 0.96 | 0.89 | |
| STDEV | 0.00 | 0.04 | 1507.72 | 18459.67 | 6.03 | 12.07 | 0.00 | 0.00 | |
| % RSD | 0.02 | 1.23 | 0.13 | 1.07 | 0.16 | 0.25 | 0.31 | 0.14 | |
Table 13: Standard results of Valsartan and Lisinopril.
|
Parameter |
Valsartan |
Lisinopril |
Acceptance criteria |
|
Retention time (min) |
2.66 |
3.424 |
± 1.0 |
|
Theoretical plate |
3600 |
4704 |
?3000 |
|
Tailing factor |
0.96 |
0.89 |
?1.50 |
|
%RSD |
0.13 |
1.07 |
?2.00 |
Table 14: System suitability data of valsartan and lisinopril.
Result: Results of system suitability study are summarized in the above table. Five consecutive injections of the standard solution showed uniform retention time, theoretical plate count, tailing factor and resolution for both the drugs which indicate a good system for analysis.
Estimation of valsartan and lisinopril in tablet dosage form
Two formulation of pill were selected for trying out the suitability of the proposed approach to estimate valsartan and lisinopril in tablet formula. Twenty pills had been weighed and powered. An accurately weighed portion of this powder equivalent to 50 mg of valsartan and lisinopril changed into transferred right into a 100 ml volumetric flask and dissolved in 50 ml of a 35:65 v/v combination of phosphate buffer and methanol. The content material of the flask has been sonicated for 15 min to make sure complete solubility of the drug. The quantity was made up with the diluents and the solution turned into filtered via a 0.45 μ membrane filter out. This solution containing 80 μg/ml valsartan and 10 μg/ml lisinopril become injected into the column six times. The average top vicinity of the drug changed into computed from the chromatograms and the quantity of the drugs gift in the tablet dosage form became calculated through the use of the regression equation obtained for the pure drug. The applicable outcomes are provided in desk beneath (Table 15).
| Formulation | Label claim | Amount found | % Amount found | |||
| Valsartan | Lisinopril | Valsartan | Lisinopril | Valsartan | Lisinopril | |
| Formulation 1 | 80 | 10 | 80.003 | 10.006 | 100.003 | 100.06 |
| Formulation 2 | 80 | 10 | 79.996 | 9.998 | 99.995 | 99.98 |
Table 15: Assay and recovery studies.
Discussion
The present experimental investigation reported in this thesis is to develop analytical method development and validation as per the ICH guidelines which are recommended for the analytical method validation. Analytical method developments were carried out for the simultaneous estimation of active pharmaceutical ingredients and its combined formulated dosage form. Valsartan and Lisinopril was the selected combination, formulated in the F and D department of Nivix Pharmaceuticals as a trials product (80 mg of valsartan and 10 mg of lisinopril). The selected drug was used for the simultaneous analysis and validation by reverse phase liquid chromatography.
The simultaneous estimation of valsartan and lisinopril in drug product was done by liquid chromatography and the chromatographic separation was achieved on C18 column (X-Terra RP-18 150*4.6 mm) column at ambient temperature. The separation achieved employing a mobile phase consists of 0.1%v/v Formic acid in water: Methanol (25:75%v/v). The flow rate was 0.8 ml/min and ultra violet detector at 237 nm. The average retention time for valsartan and lisinopril found to be 2.66 min and 3.424 min. The % purity of valsartan and lisinopril was found to be 99.16 and 99.33 respectively.
Conclusion
The present study successfully developed and validated a robust, reliable, and precise Reverse-Phase High-Performance Liquid Chromatography (RP-HPLC) method for the simultaneous estimation of valsartan and lisinopril in bulk and pharmaceutical dosage forms. The method demonstrated excellent specificity, linearity, accuracy, precision, and robustness, with retention times of 2.664 and 3.423 minutes, respectively. Recovery rates were within the acceptable range (99-100%), and %RSD values were consistently below the threshold, confirming method reproducibility. The optimized chromatographic conditions employing a phosphate buffer (pH 2.8) and methanol in a 35:65 v/v ratio, a flow rate of 0.8 mL/min, and a detection wavelength of 237 nm enabled clear resolution and quantification of both drugs without interference. This method complies with ICH and FDA guidelines for method validation and is suitable for routine quality control, laboratory analysis, and in-house drug evaluation, ensuring high analytical performance in pharmaceutical environments.
Acknowledgement
This project work would not have been possible without the support, endurance, guidance and supervision of the following people. It is them that owe my deepest gratitude.
I would like to express my humble gratitude to my supervisor Miss Parul Bisht, Ass. Prof., Department of Pharmacy, Shree Dev Bhoomi Institute of Education, Science and Technology for the proper guidance and continuous correspondence which helped me to work efficiently.
I will remain indebted to Prof. Dr. Shiva Nanda Patil Principal and Ass. Prof. Ms Santoshi Shah program co-ordinator Department of Pharmacy, Quality Assurance, Shree Dev Bhoomi Institute of Education, Science and Technology, Poudha, Dehradhun, Uttarakhand, India for their best guidance and giving me permission for access to use the college laboratory and allowing me conduct my thesis work on this topic.
Equal gratitude and appreciation goes to Ass. Prof. Ms Rita Saini, Ass. Prof. Anupriya Adhikari and all other Lectures of Department of Pharmacy, Shree Dev Bhoomi Institute of Education, Science and Technology for their support and suggestions for the completion of my research work.
I would like to express my sincere gratitude to Mr. Prakash Gyawali, Managing Director of Biogain Remedies Pvt. Ltd., Tollotama-14, Rupandehi, Nepal for providing raw materials as a gift and accessing the quality control department for the thesis project.
I am very thankful to Mr. Sushan Shrestha senior officer formulation and Development Department of Nivix Pharmaceutical, to my seniors Mr. Bidur Chapagain and Ms Suchitra Panjiyar for their support and suggestions during my research work.
I would like to express thanks to my colleagues Mr. Arjun Saraf, Miss Sangam Jadaun and all my batchmates for their help and support during thesis work and for their valuable support during my research work.
Last but not the least I am thankful to all the people who support me directly and indirectly to complete this project work in timely manner.
References
- Beckett AH, et al. 4th Edition. CBS publisher and distributors. New Delhi, India. 1986.
- David CL. 6th Edition. Black well publishing. London, England. 1994.
- LG C. 2nd Edition. Marcel Dekker. New York, USA. 1996.
- Chatwal GR. 5th Edition. Himalaya Publishing House. New Delhi, India. 2008.
- Jack Cazes. 3rd Edition, CRC Press. Boca Raton, Florida. 2004:1064.
- Siddiqui Masoom Raza, et al. Arab J Chem. 2013;10:1-12.
- Willard HH, et al. 7th Edition. CBS Publishers and Distributors. New Delhi, India. 1988.
- Bai RRM. Res Rev J Pharm Qual Assur. 2016;2(1).
- Gupta V, et al. Int Res J Pharm Appl Sci. 2012;2(4):17-25.
- Chauhan Ashish, et al. J Anal Bioanal Tech. 2015:233-238.
- Rajani, et al. Curr Pharma Res. 2013;3(2):855-870.
- Shabir G, et al. J Liq Chromatogr Relat Technol. 2007;30(3):311-333.
- Thompson M, et al. Int Union Pure Appl Chem. 2002;74(5):835-855.
- Saitoh M, et al. ESC Heart Fail. 2017;4(4):448-457.
- Chandra A, et al. JAMA cardiol. 2018;3(6):498-505.
- Cwynar M, et al. J Hum Hypertens. 2015;29(10):583-591.
- Iekushi K, et al. Hypertens. 2007;49(6):1409-1414.
- Lueder TG, et al. Cardiovasc Drugs Ther. 2013;27:171-179.
- Begum S, et al. Asian J Pharm Anal. 2021;11(2):133-138.
- Buddhadev S, et al. Int J Innov Pharm Sci Res. 2015;3(3):212-220.