Original Articles: 2022 Vol: 14 Issue: 11
Ab initio and DFT Study of Thymine and Water Complexes
Brijesh Kumar Sharma, AchchheLal, Devendra Kumar Singh*
Department of Physics, Udai Pratap (Autonomous) College, Varanasi, Uttar Pradesh, India
- Corresponding Author:
- Devendra Kumar Singh
Department of Physics
Udai Pratap (Autonomous) College
Varanasi, Uttar Pradesh, India
Received: 20-Apr-2022, Manuscript No. JOCPR-22-68172; Editor assigned: 23-Apr-2022, PreQC No. JOCPR-22-68172 (PQ); Reviewed: 07-May-2022, QC No. JOCPR-20-68172; Revised: 21-Sep-2022, QI No. JOCPR-22-68172, Manuscript No. JOCPR-22-68172 (R); Published: 19-Oct-2022
Abstract
The optimized geometries of thymine and all the three isomers of thymine-water complex, isomer-I, isomer-II and isomer-III have been obtained using Ab initio method MP2 and DFT methods B3LYP, X3LYP and B3PW91 with 6- 311++G (d,p) basis set. Structural parameters of the optimized geometries, total energies and the APT charges of thymine and all the three isomers of the thymine-water complex have been computed. Frequency calculations are carried out on each optimized structure using DFT methods and their IR and Raman spectra have been discussed. The calculated frequencies of the thymine are found to be in good agreement with the experimental values in most of the cases. We show that addition of water molecules in thymine, the strength of the binding energy decreases i.e. stability increases.
Keywords
Thymine, MP2, DFT, B3LYP, Optimized geometry
Introduction
Adenine, cytosine, guanine and thymine are the nucleobase that form the Nucleic Acid (NA) base pairs (guanine with cytosine, adenine with thymine) in DNA. Thymine is one of the pyrimidine bases, along with cytosine that makes hydrogen bonds with adenine (6-amino purine) in normal Waston-Crick base pairing. The pair wise creation of the bases occurs due to the formation of hydrogen bonds. The building by minor tautomers of NA bases of non-standard base pairs may lead to changes in the genetic code [1,2]. The perusal of the dynamics of these molecules and molecular structure can help to understand and explain some processes in biological systems. Recently, there has been an increasing interest in studying DNA damage, which may cause various diseases such as cancer [3-7]. This intellect is of great interest due to its importance for developing pharmacological substances and developing therapies against viral infections, cancer, mol formations and so on. In a recent work, the proton affinity of the two oxygen atoms and the deprotonation enthalpies of the two NH bonds of thymine have been computed using the DFT methods employing B3LYP, X3LYP and B3PW91 functional in conjunction with 6-311++G (d,p) basis set. It has been suggested that the energies of the three stable thymine-water complexes depend not only on the proton accepting ability of the oxygen atom but also on the ability of NH groups to donate proton [8]. To establish more general correlations between the acidity and basicity of an amphoteric molecule and its hydrogen bonding ability, the energy of the thymine-water complexes are computed in this work using the DFT (B3LYP) and a 6-311++G (d,p) basis set. Use of an suitable DFT functional combined with an appropriate basis set reproduces the energy of the complex very well; in fact, the energy and the intermolecular distance obtained for the thymine-water interaction are comparable with thymine calculated at the DFT (B3LYP) and a 6-311++G (d,p) basis set. For proper use of the vibrational spectra of nucleic acid bases in biophysical research, authentic knowledge of the normal mode of each vibration for each relevant IR or Raman band is essential. Three stable structures for the thymine-water complex have been studied in this work. The three thymine water isomers obtained here have been reported earlier by Chandra, et al. [9]. Optimized geometries of all the three isomers of thymine-water complex have been obtained at MP2/6-311++G (d,p), B3LYP/6-311++G (d,p), X3LYP/6-11++G (d,P) and B3PW91/6-11++G (d,p) levels of theory. Structural parameters of the optimized geometries, total energies and the APT charges of the thymine-water complex have been computed. The optimized bond length and bond angles are in agreement with the corresponding experimental results. In this work a study of change in structures and spectra of possible hydrogen bonded thymine-water complexes have been done and these have been compared with that of free thymine. We show that addition of water molecules in thymine, the strength of the binding energy decreases i.e. stability increases.
Materials and Methods
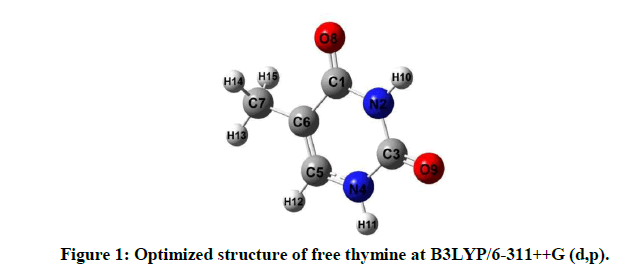
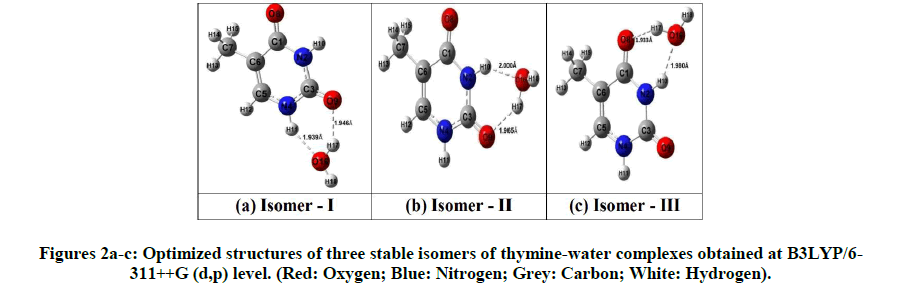
The vibrational spectra and ground state geometries for free thymine and its hydrogen bonded complexes with molecules of water have been optimized using the Ab initio method: (i) MP2 and hybrid Density Functional Theory (DFT) methods (ii) B3LYP which uses parameter Becke’s functional three with nonlocal correlation provided by Lee-Young-Parr expression with 6-311++G (d,p) basis set [10-14]. The total energies, structural parameters of the optimized geometries and the APT charges of isomers have been computed using DFT methods only. For all computational calculations, we have used Gaussian 09 package of programs [15]. Initially, Ab initio calculations were done at MP2/6-311++G (d,p) level. The optimized geometry at the MP2/6-311++G (d,p) level was taken as the input structure for the DFT calculation using B3LYP/6-311++G (d,p) level. Similarly, the optimized geometry at the B3LYP/6-311++G (d,p) level was used as the input structure for the calculation at the X3LYP/6-11++G (d,p) and B3PW91/6-11++G (d,p) level. The geometries were optimized by minimizing the energies without imposing any constraint on the geometry. It is widely accepted that this level of theory is sufficient to reliably predict molecular geometries of the hydrogen bonded systems. The optimized structures of free thymine and three isomers at B3LYP/6-311++G (d,p) along with atomic numbering have been shown in Figures 1 and 2a-c respectively.
Results and Discussion
Optimized Geometry of Thymine
The geometry optimization and charge distribution of thymine have been calculated in their ground state, at various levels of theory using the Gaussian 09 computer code. The total energy of thymine at MP2/6-311++G (d,p), B3LYP/6-311++G (d,p), X3LYP/6-311++G (d,p) and B3PW91/6-311++G (d,p) levels are found to be -453.05193380 a.u., -454.27574640 a.u., -454.08723750 a.u. and -454.09637532 a.u. respectively as listed in Table 1.
Molecular Geometry
The calculated optimized molecular energies, dipole moments, zero point vibrational energies and thermodynamic function using Ab initio method MP2 and DFT methods B3LYP, X3LYP and B3PW91 method with 6-311++G (d,p) basis set for thymine and all the three isomers of thymine-water, viz. isomer-I, isomer-II and isomer-III are shown in Table 1. The isomer-I is most stable having least optimized energy while isomer-II and isomer-III are found to have higher energies in all cases. The dipole moment of isomer-I is least and that of isomer-III is largest therefore isomer-III is more polar than the other two isomers.
| S. No. |
Species | Total energies E (hartree) |
Zero point vibrational energy (J/mol) |
Dipole moment (Debye) |
Volume molar heat capacity (C,) (cal/mol K) |
Entropy S (cal/mol K) |
|---|---|---|---|---|---|---|
| MP2/6-311++G (d,p) | ||||||
| 1 | Thymine | -453.05193380 | 297752.5 | 4.3179 | 28.762 | 87.289 |
| Thymine-water | ||||||
| 1 | Isomer-I | -529.34490302 | - | 4.2550 | ||
| 2 | Isomer -II | -529.34196051 | - | 5,1000 | - | - |
| 3 | Isomer-III | -529.34231448 | - | 4.7639 | - | - |
| B3LYP/6-311++G (d,p) | ||||||
| 1 | Thymine | -454.27574640 | 299866.2 | 4.5316 | 29,465 | 87,205 |
| Thymine-water | ||||||
| 1 | Isomer-I | -530.75078380 | 364093.0 | 3.7693 | 37.143 | 99.359 |
| 2 | Isomer -II | -530.74788215 | 364685.1 | 4.8116 | 39.195 | 102.130 |
| 3 | Isomer-III | -530.74849881 | 364839.0 | 4.5653 | 39.053 | 101.965 |
| X3LYP/6-311++G (d,p) | ||||||
| 1 | Thymine | -454.08723750 | 300512.5 | 4.5392 | 29.398 | 87.140 |
| Thymine-water | ||||||
| 1 | Isomer-I | -530.53364363 | 364902.4 | 3.7786 | 37.048 | 99.182 |
| 2 | Isomer -II | -530.53070011 | 365495.0 | 4.8088 | 39.103 | 101.945 |
| 3 | Isomer-III | -530.53131129 | 365634.9 | 4.5609 | 38.965 | 101,803 |
| B3PW91/6-311++G (d,p) | ||||||
| 1 | Thymine | -454.09637532 | 301184.1 | 4.4971 | 29.388 | 87,239 |
| Thymine-water | ||||||
| 1 | Isomer-I | -530.53995348 | 365616.3 | 3.7575 | 37,047 | 99,321 |
| 2 | Isomer -II | -530.53714789 | 366327.8 | 4.8611 | 39,058 | 101,941 |
| 3 | Isomer-III | -530.53782868 | 366607.7 | 4.6350 | 38,868 | 101,567 |
Table 1: Calculated energies, dipole moment, zero point vibrational energy and thermodynamic functions obtained at different levels.
Structural Parameters
The optimized geometrical parameters namely bond length (Å) and bond angles (in degree) of the thymine and all the three isomers of thymine-water, isomer-I, isomer-II and isomer-III computed using Ab initio method MP2 and DFT method B3LYP, X3LYP and B3PW91 functional with 6-311++G (d,p) basis set are reported along with corresponding experimental value in Tables 2-5. We see that the calculated bond lengths are very close to experimental values while calculated using B3PW91/6-311++G (d,p) and bond angles are very close to experimental values while calculated using B3LYP/6-311++G (d,p).
| Parameters | Experimental | Free thymine |
Isomer 1 | Isomer 2 | Isomer 3 |
|---|---|---|---|---|---|
| Bond length | |||||
| C1-N2 | 1.413 | 1.404 | 1.407 | 1.405 | 1.396 |
| N2-C3 | 1.345 | 1.387 | 1.382 | 1.378 | 1.387 |
| C3-N4 | 1.314 | 1.386 | 1.377 | 1.382 | 1.389 |
| N4-C5 | 1.408 | 1.380 | 1.379 | 1.382 | 1.378 |
| C5-C6 | 1.369 | 1.357 | 1.358 | 1.356 | 1.357 |
| C6-C7 | 1.522 | 1.499 | 1.499 | 1.498 | 1.499 |
| C1-O8 | 1.193 | 1.223 | 1.223 | 1.222 | 1.232 |
| C3-O9 | 1.246 | 1.218 | 1.228 | 1.227 | 1.217 |
| N2-H10 | - | 1.015 | 1.015 | 1.024 | 1.024 |
| N4-H11 | - | 1.010 | 1.020 | 1.010 | 1.011 |
| C5-H12 | - | 1.086 | 1.086 | 1.086 | 1.086 |
| C7-H13 | - | 1.093 | 1.093 | 1.093 | 1.093 |
| C7-H14 | - | 1.094 | 1.094 | 1.093 | 1.093 |
| C7-H15 | - | 1.094 | 1.094 | 1.093 | 1.093 |
| O16-H17 | - | - | 0.97 | 0.968 | 0.959 |
| O16-H18 | - | - | 0.959 | 0.959 | 0.969 |
| H11-O16 | - | - | 1.937 | - | - |
| O9-H17 | - | - | 1.983 | 2.003 | - |
| O16-H10 | - | - | - | 1.982 | 1.97 |
| O8-H17 | - | - | - | - | 1.974 |
| Bond angles | |||||
| O9-C3-N4 | 122 | 123 | 124 | 122 | 123 |
| O9-C3-N2 | 121 | 124 | 123 | 124 | 124 |
| N4-C3-N2 | 118 | 112 | 113 | 113 | 113 |
| C3-N4-C5 | 123 | 123 | 123 | 123 | 124 |
| N4-C5-C6 | 120 | 122 | 123 | 122 | 122 |
| C5-C6-C7 | 112 | 123 | 124 | 124 | 124 |
| C5-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C7-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C6-C1-N2 | 114 | 114 | 114 | 115 | 115 |
| C6-C1-O8 | 125 | 124 | 125 | 124 | 123 |
| O8-C1-N2 | 121 | 120 | 120 | 121 | 121 |
| C1-N2-C3 | 126 | 128 | 128 | 127 | 127 |
| H17-O16-H18 | - | - | 105 | 105 | 105 |
| N4-H11-O16 | - | - | 145 | - | - |
| H11-O16-H17 | - | - | 86 | - | - |
| O16-H17-O9 | - | - | 141 | 140 | - |
| H17-O9-C3 | - | - | 108 | 109 | - |
| N2-H10-O16 | - | - | - | 144 | 144 |
| H10-O16-H17 | - | - | - | 87 | 138 |
| O16-H17-O8 | - | - | - | - | 142 |
| C1-O8-H17 | - | - | - | - | 111 |
Table 2: Optimized bond lengths (Å) and bond angles (in degree) of free thymine and thymine-water complexes at MP2/6-311++G (d,p) level.
| Parameters | Experimental | Free thymine | Isomer 1 | Isomer 2 | Isomer 3 |
|---|---|---|---|---|---|
| Bond length | |||||
| C1-N2 | 1.413 | 1.407 | 1.409 | 1.407 | 1.396 |
| N2-C3 | 1.345 | 1.384 | 1.379 | 1.375 | 1.384 |
| C3-N4 | 1.314 | 1.387 | 1.378 | 1.382 | 1.391 |
| N4-C5 | 1.408 | 1.380 | 1.377 | 1.381 | 1.376 |
| C5-C6 | 1.369 | 1.349 | 1.351 | 1.348 | 1.350 |
| C6-C7 | 1.522 | 1.500 | 1.499 | 1.499 | 1.500 |
| C1-O8 | 1.193 | 1.217 | 1.217 | 1.216 | 1.229 |
| C3-O9 | 1.246 | 1.213 | 1.225 | 1.225 | 1.212 |
| N2-H10 | - | 1.013 | 1.012 | 1.023 | 1.024 |
| N4-H11 | - | 1.009 | 1.019 | 1.009 | 1.009 |
| C5-H12 | - | 1.083 | 1.083 | 1.083 | 1.083 |
| C7-H13 | - | 1.091 | 1.092 | 1.092 | 1.092 |
| C7-H14 | - | 1.093 | 1.093 | 1.093 | 1.093 |
| C7-H15 | - | 1.093 | 1.093 | 1.093 | 1.093 |
| O16-H17 | - | - | 0.974 | 0.973 | 0.975 |
| O16-H18 | - | - | 0.96 | 0.961 | 0.961 |
| H11-O16 | - | - | 1.939 | - | - |
| O9-H17 | - | - | 1.946 | 1.965 | - |
| O16-H10 | - | - | - | 2 | 1.98 |
| O8-H17 | - | - | - | - | 1.933 |
| Bond angles | |||||
| O9-C3-N4 | 122 | 123 | 123 | 122 | 123 |
| O9-C3-N2 | 121 | 124 | 123 | 124 | 124 |
| N4-C3-N2 | 118 | 112 | 113 | 114 | 113 |
| C3-N4-C5 | 123 | 123 | 123 | 123 | 124 |
| N4-C5-C6 | 120 | 122 | 123 | 122 | 122 |
| C5-C6-C7 | 112 | 123 | 124 | 124 | 124 |
| C5-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C7-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C6-C1-N2 | 114 | 114 | 114 | 115 | 116 |
| C6-C1-O8 | 125 | 125 | 125 | 125 | 124 |
| O8-C1-N2 | 121 | 120 | 120 | 120 | 120 |
| C1-N2-C3 | 126 | 128 | 128 | 127 | 127 |
| H17-O16-H18 | - | - | 108 | 107 | 107 |
| N4-H11-O16 | - | - | 143 | - | - |
| H11-O16-H17 | - | - | 88 | - | - |
| O16-H17-O9 | - | - | 140 | 142 | - |
| H17-O9-C3 | - | - | 110 | 110 | - |
| N2-H10-O16 | - | - | - | 142 | 143 |
| H10-O16-H17 | - | - | - | 86 | 84 |
| O16-H17-O8 | - | - | - | 143 | |
| C1-O8-H17 | - | - | - | 112 | |
Table 3: Optimized bond lengths (Å) and bond angles (in degree) of free thymine and thymine-water complexes at B3LYP/6-311++G (d,p) level.
| Parameters | Experimental | Free thymine | Isomer 1 | Isomer 2 | Isomer 3 |
|---|---|---|---|---|---|
| Bond length | |||||
| C1-N2 | 1.413 | 1.406 | 1.407 | 1.405 | 1.395 |
| N2-C3 | 1.345 | 1.383 | 1.378 | 1.374 | 1.383 |
| C3-N4 | 1.314 | 1.386 | 1.377 | 1.380 | 1.389 |
| N4-C5 | 1.408 | 1.379 | 1.376 | 1.381 | 1.375 |
| C5-C6 | 1.369 | 1.348 | 1.350 | 1.347 | 1.349 |
| C6-C7 | 1.522 | 1.499 | 1.499 | 1.498 | 1.499 |
| C1-O8 | 1.193 | 1.216 | 1.216 | 1.215 | 1.228 |
| C3-O9 | 1.246 | 1.212 | 1.224 | 1.224 | 1.212 |
| N2-H10 | - | 1.012 | 1.012 | 1.023 | 1.024 |
| N4-H11 | - | 1.008 | 1.019 | 1.008 | 1.009 |
| C5-H12 | - | 1.083 | 1.083 | 1.083 | 1.083 |
| C7-H13 | - | 1.092 | 1.091 | 1.092 | 1.092 |
| C7-H14 | - | 1.093 | 1.093 | 1.093 | 1.093 |
| C7-H15 | - | 1.093 | 1.093 | 1.093 | 1.093 |
| O16-H17 | - | - | 0.974 | 0.973 | 0.974 |
| O16-H18 | - | - | 0.959 | 0.960 | 0.960 |
| H11-O16 | - | - | 1.930 | - | - |
| O9-H17 | - | - | 1.938 | 1.955 | - |
| O16-H10 | - | - | - | 1.990 | 1.970 |
| O8-H17 | - | - | - | - | 1.924 |
| Bond angles | |||||
| O9-C3-N4 | 122 | 123 | 123 | 122 | 123 |
| O9-C3-N2 | 121 | 124 | 123 | 124 | 124 |
| N4-C3-N2 | 118 | 112 | 113 | 114 | 113 |
| C3-N4-C5 | 123 | 123 | 123 | 123 | 124 |
| N4-C5-C6 | 120 | 122 | 123 | 122 | 122 |
| C5-C6-C7 | 112 | 123 | 124 | 124 | 124 |
| C5-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C7-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C6-C1-N2 | 114 | 114 | 114 | 115 | 116 |
| C6-C1-O8 | 125 | 125 | 125 | 124 | 124 |
| O8-C1-N2 | 121 | 120 | 120 | 120 | 121 |
| C1-N2-C3 | 126 | 128 | 128 | 127 | 127 |
| H17-O16-H18 | - | - | 108 | 107 | 107 |
| N4-H11-O16 | - | - | 143 | - | - |
| H11-O16-H17 | - | - | 88 | - | - |
| O16-H17-O9 | - | - | 140 | 142 | - |
| H17-O9-C3 | - | - | 110 | 110 | - |
| N2-H10-O16 | - | - | - | 142 | 143 |
| H10-O16-H17 | - | - | - | 86 | 84 |
| O16-H17-O8 | - | - | - | - | 143 |
| C1-O8-H17 | - | - | - | - | 112 |
Table 4: Optimized Bond lengths (Å) and Bond angles (in Degree) of free thymine and thymine-water complexes at X3LYP/6-311++G (d,p) level.
| Parameters | Experimental | Free thymine | Isomer 1 | Isomer 2 | Isomer 3 |
|---|---|---|---|---|---|
| Bond length | |||||
| C1-N2 | 1.413 | 1.402 | 1.404 | 1.402 | 1.391 |
| N2-C3 | 1.345 | 1.380 | 1.374 | 1.371 | 1.380 |
| C3-N4 | 1.314 | 1.383 | 1.374 | 1.377 | 1.387 |
| N4-C5 | 1.408 | 1.375 | 1.372 | 1.376 | 1.371 |
| C5-C6 | 1.369 | 1.349 | 1.350 | 1.347 | 1.350 |
| C6-C7 | 1.522 | 1.494 | 1.494 | 1.494 | 1.495 |
| C1-O8 | 1.193 | 1.215 | 1.215 | 1.214 | 1.227 |
| C3-O9 | 1.246 | 1.211 | 1.223 | 1.22 | 1.211 |
| N2-H10 | - | 1.012 | 1.012 | 1.024 | 1.025 |
| N4-H11 | - | 1.008 | 1.020 | 1.008 | 1.008 |
| C5-H12 | - | 1.084 | 1.084 | 1.084 | 1.084 |
| C7-H13 | - | 1.092 | 1.092 | 1.092 | 1.092 |
| C7-H14 | - | 1.094 | 1.094 | 1.094 | 1.094 |
| C7-H15 | - | 1.094 | 1.094 | 1.094 | 1.094 |
| O16-H17 | - | - | 0.973 | 0.972 | 0.974 |
| O16-H18 | - | - | 0.958 | 0.959 | 0.959 |
| H11-O16 | - | - | 1.914 | - | - |
| O9-H17 | - | - | 1.933 | 1.951 | - |
| O16-H10 | - | - | - | 1.976 | 1.954 |
| O8-H17 | - | - | - | - | 1.916 |
| Bond angles | |||||
| O9-C3-N4 | 122 | 123 | 123 | 122 | 123 |
| O9-C3-N2 | 121 | 124 | 123 | 124 | 124 |
| N4-C3-N2 | 118 | 112 | 113 | 114 | 113 |
| C3-N4-C5 | 123 | 123 | 123 | 123 | 124 |
| N4-C5-C6 | 120 | 122 | 123 | 122 | 122 |
| C5-C6-C7 | 112 | 123 | 124 | 124 | 124 |
| C5-C6-C1 | 119 | 117 | 118 | 118 | 118 |
| C7-C6-C1 | 119 | 118 | 118 | 118 | 118 |
| C6-C1-N2 | 114 | 114 | 114 | 115 | 116 |
| C6-C1-O8 | 125 | 125 | 125 | 124 | 124 |
| O8-C1-N2 | 121 | 120 | 120 | 120 | 121 |
| C1-N2-C3 | 126 | 128 | 128 | 127 | 127 |
| H17-O16-H18 | - | - | 108 | 107 | 107 |
| N4-H11-O16 | - | - | 143 | - | - |
| H11-O16-H17 | - | - | 88 | - | - |
| O16-H17-O9 | - | - | 141 | 143 | - |
| H17-O9-C3 | - | - | 110 | 109 | - |
| N2-H10-O16 | - | - | - | 143 | 143 |
| H10-O16-H17 | - | - | - | 85 | 84 |
| O16-H17-O8 | - | - | - | - | 143 |
| C1-O8-H17 | - | - | - | - | 112 |
Table 5: Optimized Bond lengths (Å) and Bond angles (in Degree) of free Thymine and Thymine-Water complexes at B3PW91/6-311++G (d,p) level.
Atomic Polar Tensor (APT) charges
The Atomic Polar Tensor (APT) charges for the thymine and thymine-water complexes computed at using DFT method B3LYP, X3LYP and B3PW91 method with 6-311++G (d,p) basis sets are collected in Tables 6-8 for atomic numbering scheme, see Figure 1 and Figure 2a-c respectively. In terms of the charge ofelectron 1e=1.602188 × 10-19 C from Tables 6-8, we see that due to high negativity of the respective atomscompared to the other atoms result enhancement of the bond length. In free thymine, we see that the bondlength of magnitudes of the bond lengths between the pair (C1-O8) and (C3-O9) are found to be differed dueto O9 is more negative than the O8. Thus O9 attracts more to C atoms than O8 which gives difference intheir bond lengths. Hence the bond length of (C3=O9) is shorter than the bond length of (C1=O8) similarly,with the help of APT (Atomic Polar Tensor) charges we can explain the difference in the bond length ofothers pairs e.g. C1-N2 and N2-C3; C3-N4 and N4-C5; C5-C6 and C6-C7.
| Atoms | Free thymine | Isomer I | Isomer II | Isomer III |
|---|---|---|---|---|
| C1 | 1.134 | 1.135 | 1.139 | 1.153 |
| N2 | -0.727 | -0.727 | -0.789 | -0.786 |
| C3 | 1.335 | 1.337 | 1.351 | 1.339 |
| N4 | -0.737 | -0.791 | -0.747 | -0.733 |
| C5 | 0.459 | 0.488 | 0.439 | 0.459 |
| C6 | -0.296 | -0.305 | -0.279 | -0.32 |
| C7 | 0.086 | 0.086 | 0.083 | 0.0893 |
| O8 | -0.837 | -0.85 | -0.827 | -0.927 |
| O9 | -0.922 | -1.01 | -1.005 | -0.912 |
| H10 | 0.219 | 0.221 | 0.346 | 0.348 |
| H11 | 0.244 | 0.38 | 0.245 | 0.244 |
| H12 | 0.053 | 0.055 | 0.052 | 0.053 |
| H13 | -0.008 | -0.008 | -0.01 | -0.009 |
| H14 | -0.001 | -0.003 | -0.001 | -0.002 |
| H15 | -0.001 | -0.003 | 0.001 | -0.001 |
| O16 | - | -0.723 | -0.669 | -0.682 |
| H17 | - | 0.288 | 0.399 | 0.272 |
| H18 | - | 0.43 | 0.273 | 0.416 |
Table 6: APT charges at various atomic sites of free thymine molecule and thymine- water complexes at B3LYP/6-311++G (d,p) level.
| Atoms | Free thymine | Isomer I | Isomer II | Isomer III |
|---|---|---|---|---|
| C1 | 1.140 | 1.140 | 1.145 | 1.160 |
| N2 | -0.731 | -0.731 | -0.794 | -0.791 |
| C3 | 1.341 | 1.343 | 1.357 | 1.345 |
| N4 | -0.742 | -0.796 | -0.752 | -0.738 |
| C5 | 0.462 | 0.492 | 0.442 | 0.462 |
| C6 | -0.299 | -0.308 | -0.282 | -0.323 |
| C7 | 0.085 | 0.085 | 0.082 | 0.088 |
| O8 | -0.841 | -0.853 | -0.831 | -0.932 |
| O9 | -0.926 | -1.015 | -1.009 | -0.916 |
| H10 | 0.221 | 0.222 | 0.349 | 0.351 |
| H11 | 0.245 | 0.383 | 0.246 | 0.245 |
| H12 | 0.054 | 0.0558 | 0.053 | 0.054 |
| H13 | -0.008 | -0.007 | -0.01 | -0.008 |
| H14 | -0.001 | -0.002 | 0.001 | -0.001 |
| H15 | -0.001 | -0.002 | 0.001 | -0.001 |
| O16 | - | -0.728 | -0.674 | -0.687 |
| H17 | - | 0.290 | 0.401 | 0.274 |
| H18 | - | 0.432 | 0.275 | 0.418 |
Table 7: APT charges at various atomic sites of free thymine molecule and thymine- water complexes at X3LYP/6-311++G (d,p) level.
| Atoms | Free thymine | Isomer I | Isomer II | Isomer III |
|---|---|---|---|---|
| C1 | 1.128 | 1.128 | 1.132 | 1.146 |
| N2 | -0.725 | -0.724 | -0.793 | -0.790 |
| C3 | 1.329 | 1.331 | 1.344 | 1.333 |
| N4 | -0.737 | -0.793 | -0.745 | -0.732 |
| C5 | 0.454 | 0.483 | 0.433 | 0.454 |
| C6 | -0.294 | -0.303 | -0.276 | -0.318 |
| C7 | 0.069 | 0.069 | 0.065 | 0.071 |
| O8 | -0.833 | -0.845 | -0.822 | -0.927 |
| O9 | -0.919 | -1.010 | -1.004 | -0.908 |
| H10 | 0.222 | 0.223 | 0.357 | 0.360 |
| H11 | 0.247 | 0.390 | 0.248 | 0.247 |
| H12 | 0.247 | 0.057 | 0.054 | 0.056 |
| H13 | -0.004 | -0.003 | -0.005 | -0.004 |
| H14 | 0.004 | 0.001 | 0.005 | 0.002 |
| H15 | 0.004 | 0.001 | 0.005 | 0.004 |
| O16 | - | -0.731 | -0.675 | -0.689 |
| H17 | - | 0.291 | 0.402 | 0.274 |
| H18 | - | 0.434 | 0.274 | 0.421 |
Table 8: APT Charges at various atomic sites of free thymine molecule and thymine- water complexes at B3PW91/6-311++G (d,p) level.
Thermodynamic Properties
Few calculated thermo dynamical parameters such as the Zero Point Vibration Energies (ZPVE) the molar capacity at constant volume, the entropy and dipole moment are listed in table in the previous section. The variations in the ZPVES (Zero Point Vibration Energies) seem to be insignificant. Changes in the total entropy and the molar capacity at constant volume of thymine and thymine-water at DFT method B3LYP, X3LYP and B3PW91 method with 6-311++g (d,p) basis set are also marginal only.
Conclusion
The optimized geometries of thymine and three isomers of thymine-water complexes have been calculated employing Ab initio method MP2 and DFT method B3LYP, X3LYP and B3PW91 method with 6-311++G (d,p) basis set using Gaussian 09 program. Most of the geometrical parameters for thymine-water complexes remain the same as thymine except for the geometry of the site of the water bonded atom. Structural parameters of the optimized geometries, total energies and the APT charges of the thymine-water complex have been discussed in detail. From this analysis, it is noted that the theoretically calculated optimized bond lengths are comparatively larger than the experimental values because the theoretical calculations refer to isolated molecules in the gas phase while it is in the solid state for experimental results. We show that addition of water molecules in thymine, the strength of the binding energy decreases i.e. stability increases. The optimized bond length and bond angles are in agreement with the corresponding experimental results.
Acknowledgement
The authors are grateful to Secretary and Principal, Udai Pratap Autonomous College for providing the necessary facilities.
References
- Topal MD, Fresco JR. Nature. 1976;263(5575):289-293. [Crossref][Googlescholar][Indexed]
- Demple B, Harrison L. Annu Rev Biochem. 1994,63:915-948. [Crossref][Googlescholar][Indexed]
- Loft S, Poulsen HE. J Mol Med. 1996;74(6):297-312. [Crossref][Googlescholar][Indexed]
- Nakabeppu Y, Sakumi K, Sakamoto K, et al. Biol Chem. 2006;387(4):373-379. [Crossref][Googlescholar][Indexed]
- Michael BD, O'Neill P. Science. 2000;287(5458):1603-1604. [Crossref][Googlescholar][Indexed]
- Miller JH, Wilson WW, Ritchie RH. Comput Mol Biol. 1994;63:65-76. [Crossref]
- Chandra AK, Nguyen MT. J Chem Soc Faraday Trans. 1998;94(4):1277-1280. [Googlescholar]
- Chandra AK, Nguyen MT, Zeegers-Huyskens T. J Phys Chem A. 1998;102(29):6010-6016. [Crossref][Googlescholar]
- Moller C, Plesset MS. Phys Rev. 1934;46(7):618-622. [Crossref][Googlescholar]
- Becke AD. J Chem Phys. 1992;97(12):9173-9177. [Crossref][Googlescholar]
- Becke AD. J Chem Phys. 1993;98(7):5648-5652. [Crossref][Googlescholar]
- Becke AD. J Chem Phys. 1996;104(3):1040-1046. [Crossref][Googlescholar]
- Lee C, Yang W, Parr RG. Phys Rev BCondens Matter. 1988;37(2):785-789. [Crossref][Googlescholar][Indexed]
- Frisch MJ, Trucks GW, Schlegel HB, et al. Revision D.01, Gaussian Inc., Pittsburgh, PA, 2009.
- Ozeki K, Sakabe N, Tanaka J. Acta Cryst. 1969;25(6):1038-1045. [Crossref][Googlescholar]