Original Articles: 2022 Vol: 14 Issue: 7
A Quality by Design Approach: Development and Evaluation of Herbal Hydrogel
Gopi Snehal Kumar Patel1*, BA Patel2
1Department of Pharmaceutical Science, Sardar Patel University, V.V. Nagar, India
2Department of Pharmaceutical Chemistry and Analysis, Indukaka Ipcowala College of Pharmacy, New V.V. Nagar, India
- Corresponding Author:
- Gopi Snehal Kumar Patel
Department of Pharmaceutical Science,
Sardar Patel University,
V.V. Nagar,
India
Received: 25-Jul-2022, Manuscript No. JOCPR-22-28612; Editor assigned: 27-Jul-2022, PreQC No.JOCPR-22-28612(PQ); Reviewed: 11-Aug-2022, QC No. JOCPR-22-28612; Revised: 18-Aug-2022, Manuscript No.JOCPR-22-28612(R); Published: 25-Aug-2022, DOI:10.37532/09757384.2022.14(7).037
Abstract
The current study was undertaken to formulate and evaluate herbal hydrogel containing ethanolic coix seed extract. Hydrogel was prepared by ethanolic seed extract, carbapol 934p, Aloevera gel extract, Triethanolamine and methylparaben. The effects of critical parameters (concentration of Carbapol 934p and Aloevera gel extract) were investigated by executing design of experimentation using 32 factorial designs by Design Expert 12 version software. All the formulation were developed and evaluated for visual inspection, pH, viscosity, spread-ability, drug release and drug content. Optimized formulation was subjected in vitro antifungal activity against Candida albicans. It was observed that formulation variables X1: Carbapol 934p and X2: Aloevera gel extract showed significant effect on the response Y1: Viscosity (cp) and Y2: Drug release (% CADD/cm2). Stability studies conducted under accelerated condition and at room temperature were shown acceptable results. It was concluded that hydrogel containing coix ethanolic seed extract showed good consistency, spread-ability, homogeneity as well as stability. This study confirmed that quality by design is an effective approach for understanding the quality parameters for optimizing Hydrogel formulation.
Keywords
Herbal hydrogel; Candida albicans; Homogeneity; Phytoconstituents
Abbreviations
CADD: Cumulative Amount of Diffused Drug; QbD: Quality by Design; QTPP: Quality Target Product Profile; CQA: Critical Quality Attributes
Introduction
Herbal medicinal system is old and is practiced from the beginning of mankind [1]. According to an estimate of the World Health Organization (WHO), about 80% of the world population uses herbs and other traditional medicines. They are known for their safety, efficacy, cultural acceptability and lesser side effects [2].
Coix lacryma jobi L. is belonging to Poaceae or Gramineae family. Coix plant is a grass crop that is used in traditional Chinese medicine. Adlay seeds are major medicinal part that contains a range of phytoconstituents such as Polysaccharide, Coixol, Coixenolide, Protein, Lipid, Phytosterol and Polyphenols (Figures 1 and 2). Several studies have demonstrated that coix seeds have antimicrobial, anti-diabetic, anti-inflammatory, anti-obesity, anti-cancer activity and other beneficial effect on humans [3-5].
Topical drug delivery system widely used for skin disease like bacterial infection, fungal infection, eczema etc. Topical application of drug has advantage of directly delivered of drug to the site of action. The advantages of topic formulations are avoidance of first pass metabolism, avoid inter-intra patient variation, convenient and easy to apply etc. [6]. Hydrogels are three-dimensional, cross-linked networks of water-soluble polymers. It is a semi occlusive film over the skin and release the drug in controlled manner. Hydrogel possess degree of flexibility very similar to natural tissue, due to their significant water content, good transport properties. Hydrogel are biocompatible, easy to modify and time release growth factor [7,8].
There was no report on the formulation containing extract of Coix seed. Hence, in the present study to design, formulate and evaluate hydrogel containing ethanolic extract of adlay seed.
To document product development [ICH guideline (Q9)] report by “Quality by Design” approach for Hydrogel formulation. The objective of the product development report is to present the quality by design aspect for gel based formulations. The elements of quality by design are examined and a consistent nomenclature is proposed with the help of Quality Target Product Profile (QTPP), Critical Quality Attribute (CQA), Critical Process Parameter, Critical Material Attribute (CMA), risk assessment, and control strategy [9,10].
Quality by Design is a structured, organised method for determination the relation between factor affecting a process and the output of that process based on quality risk management, ICH guideline (ICH Q8 (R2)).
Materials and Methods
Ethanolic extract of Coix seed was purchase from Shannxi Green Bio-Engineering Co. Ltd., China. Aloevera gel extract obtained from Arogya Jyoti Pharmacy, New V.V. Nagar as a gift sample. Carbapol 934p (gel base), Triethanolamine (pH adjust) and Methyl paraben (Preservative) were used in preparation of Hydrogel.
Quality Target Product Profile (QTPP)
The Quality Target Product Profile as described in International Council for Harmonization (ICH) Q8 is an essential element of a QbD approach. The QTPP includes all product attributes that are needed to ensure equivalent safety and efficacy. QTPP for herbal hydrogel has been developed by taking into account the important drug product quality attributes elements (Table 1).
| Parameters | QTPP element | Justification | Critical quality attributes | ||
|---|---|---|---|---|---|
| Low | Medium | High | |||
| Dosage form | Hydrogel | Topical application | √ | ||
| Dosage design | Controlled release | Better for topic application | √ | ||
| Strength | 5% | Effective dose | √ | ||
| Assay | 99% | Effect on safety and efficacy | √ | ||
| pH | 6–7 | Compatible with skin | √ | ||
| Viscosity | Medium | Impact on drug release | √ | ||
| Spreadability | Good | Uniformity on skin | √ | ||
| Drug release | Permeation | Impact on therapeutic point on view | √ | ||
Table 1: Qtpp for hydrogel
Critical Quality Attributes (CQA)
Critical Quality Attribute (CQA) is “a physical, chemical, biological, or microbiological property or characteristic that should be within an appropriate limit, range, or distribution to ensure the desired product quality. The initial CQA were defined from QTPP to identify satisfactory quality of the product. The potential CQA of excipients required for development of herbal hydrogel [11].
Risk Assessment
To identify all the potential high risk factors for further study, risk assessment was conducted. In the risk assessment process, Risk Priority Numbers (RPN) was mapped into three categories (high, medium and low). The initial risk assessment for critical input material and formulation components and their impact on the product quality was determined [12].
Design of Experiment (DoE)
DoE is a structured, organized method for determining the relationship between factors affecting a process and the output of that process. Design expert version 12 software (Stat-Ease Inc., Minneapolis, MN, USA) was used for formulation design. A 32 factorial design was employed where the amount of two factors were varied at three levels (-1, 0 and +1) as hypothesized by the design. In this design, two factors, Carbapol 934p and Aloevera gel extract in each in three levels (Table 2) were evaluated and experimental trials were performed in all 9 possible combinations. (Table 3) Carbapol 934p and Aloevera gel extract were selected for independent variables and viscosity and drug release were selected for dependent variables.
| Variables | Level | |||
|---|---|---|---|---|
| -1 | 0 | 1 | ||
| Independent variable | ||||
| Carbapol 934p | 1 | 1.5 | 2 | |
| Aloevera gel extract | 1 | 1.5 | 2 | |
| Dependent variable | ||||
| Viscosity | Y1=Viscosity (cp) | |||
| Drug release | Y2=Drug release (%CADD/cm2) | |||
Table 2: Selected factor combination as per 32 full factorial designs
| Ingredients | F1 | F2 | F3 | F4 | F5 | F6 | F7 | F8 | F9 |
|---|---|---|---|---|---|---|---|---|---|
| Seed extract | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 | 5 |
| Carbapol 934 p | 1.5 | 1 | 2 | 2 | 2 | 1 | 1 | 1.5 | 1.5 |
| Aloevera gel extract | 2 | 1.5 | 1.5 | 2 | 1 | 1 | 2 | 1 | 1.5 |
| Triethanolamine | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. | q.s. |
| Methyl paraben | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 | 0.2 |
| Distilled water (ml) | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 | 100 |
Table 3: Formulation trial batches of herbal hydrogel Concentration of each ingredient is expressed in % w/v
Drug Excipient Compatibility Studies
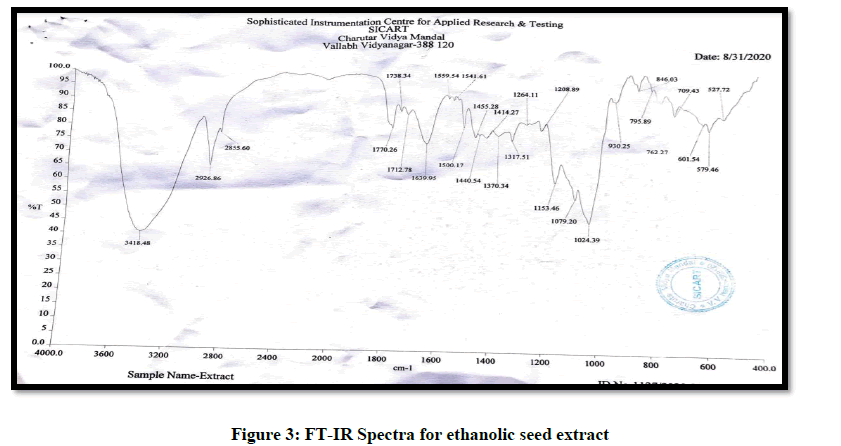
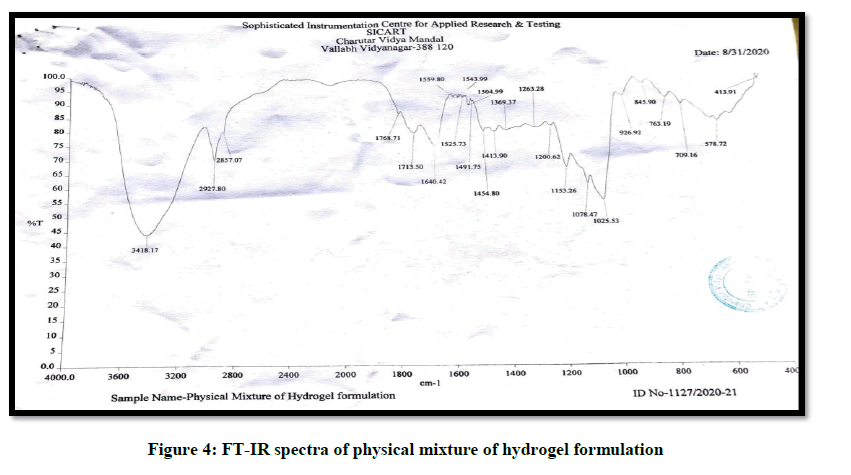
A Fourier-Transform Infrared (FT-IR) was used to identify if any interaction existed between seed extract and the excipients used. The samples were analyzed via potassium bromide pellet method in the region 4000-400 cm-1 [13] (Figures 3-5) (Table 4).
| Band assignment | Std. Range (cm-1) | Observed (cm-1) |
|---|---|---|
| 1. C-N (S) | 1350-1550 | 1024 |
| 2. C=C (S) | 1600-1475 | 1500 |
| 3. N-H (S) | 1640-1550 | 1639 |
| 4. C=O (S) | 1725-1705 | 1712 |
| 5. C-H (S) | 3130-3050 | 2926 |
Table 4: Ir spectra-interaction between seed extract and excipient
Formulation of Hydrogel
Weighed accurately amount of Carbapol 934p and dissolved in water using magnetic stirrer. Coix seed extract, Aloevera gel extract and Methylparaben were added to the gel base. Triethanolamine was added to adjust the pH of hydrogel between 6.0 to 7.0 with constant stirring.
Evaluation of Hydrogel Formulation
The formulated gel formulations were evaluated for physical examination. The characterization and response in the design of the experiments conducted on the gel preparations include pH, spreadability, homogeneity, viscosity, drug release and drug content [14-16] (Table 5).
| Run | Spreadability | pH | Viscosity (cp) | % CADD/cm2 (after 5 hrs.) | Drug content (%) |
|---|---|---|---|---|---|
| F1 | Good | 6.82 | 32645 | 52.26 | 98.86 |
| F2 | Good | 6.99 | 28923 | 62.12 | 97.16 |
| F3 | Average | 6.93 | 33249 | 52.9 | 97.12 |
| F4 | Average | 6.92 | 34920 | 50.12 | 97.27 |
| F5 | Average | 6.55 | 33712 | 51.98 | 98.26 |
| F6 | Good | 6.92 | 28092 | 62.53 | 97.78 |
| F7 | Good | 6.89 | 32590 | 52.68 | 98.51 |
Table 5: Variables and response as per design expert
Spreadability: The gel was weighed to be as high as 0.5 g and then placed on glass plate. Then, put another other glass plate above the gel mass. The gel was spread over the slides. Then gel was spread uniformly over the glass plate.
Homogeneity: After the gels have been set in container, all developed gels were tested for homogeneity by visual inspection. They were tested for their appearance and presence of any aggregates.
Viscosity: A maximum of 100 g of gel was put into a container, and then placed on a viscometer with spindle no. 64 installed. Then, the spindle was lowered onto the gel with 10 rpm speed.
Drug content: 1 g of Hydrogel was accurately weighed dissolved in 80 ml Phosphate buffer (pH 6.8). Sonicated for 10 min. and made up volume up to 100 ml with Phosphate buffer. From this 1 ml was pipetted out and diluted to 10 ml same solvent. The absorbance was measured by UV spectroscopy at 286 nm against blank.
In vitro Diffusion study
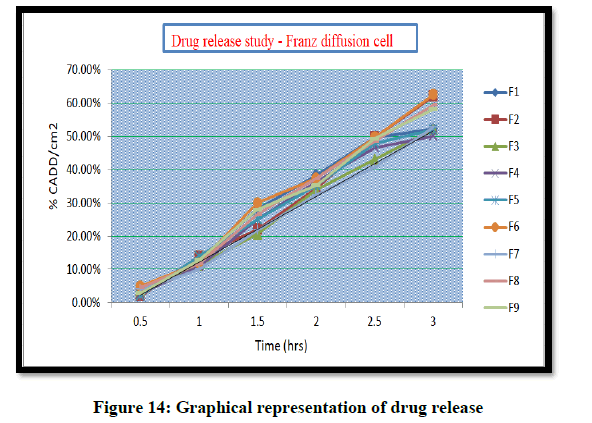
The diffusion studies of the prepared gels were carried out in Franz diffusion cell for studying the release of gels through a cellophane membrane. Place 1 gm hydrogel was taken on cellophane membrane and the diffusion studies carried out at 37 ± 1ºC using Phosphate buffer (pH 6.8) as dissolution medium. Three ml of each sample was withdrawn periodically at 1, 2, 3, 4 and 5 hr. and each sample was replaced with equal volume of dissolution medium. The samples were analyzed for the drug content by using Phosphate buffer as blank. The absorbance was analyzed by UV spectroscopy at 286 nm.
Statistical Optimization
The evaluation of the quality of fit of the model was performed by using analysis of variance (ANOVA) technique. Based on comparison of several statistical parameters the best fit model was selected, the statistical parameters included the R2-coefficient of determination, Adjusted R2, Predicted R2, p value provided by DoE software. The relationship between the dependent and independent variables was demonstrated using response surface plots i.e. Contour and 3D surface plots, these plots were used to study the effect of various factors on the response (Tables 6 and 7). Optimized formulations were run for triplicate and evaluate for pH, viscosity, drug content and dug release. (Table 8).
| Response | p-Value | R2 | Adjusted R2 | Predicted R2 |
|---|---|---|---|---|
| Y1-Viscosity | 0.0091 | 0.9932 | 0.9819 | 0.9316 |
| Y2-Drug release | 0.0085 | 0.9813 | 0.9501 | 0.7823 |
Note: p-values were less than 0.0500 indicate model terms are significant
Table 6: Statistical parameters–Analysis Of Variance (ANOVA)
| Response | Model | Polynomial equation |
|---|---|---|
| Viscosity | Quadratic model | 29887.4+1879.33 × A+1388.33 × B ± 822.5 × AB+817.333 × A^2+1564.33 × B^2 |
| Drug release | Quadratic model | 58.5389+-3.72167 × A+-3.15833 × B+1.9975 × AB ± 1.23833 × A^2 ± 2.86833 × B^2 |
Note: “×” Multiplication
Table 7: Polynomial equation
| Formulation | pH ± SD | Viscosity ± SD | Drug content (%) | % CADD/cm2 |
|---|---|---|---|---|
| F9 | 6.92 ± 0.02121 | 30123 ± 0.707107 | 98.06 ± 0.02121 | 58.25 ± 0.3606 |
Table 8: Evaluation parameters of optimized formulation
Anti-Bacterial Activity
Optimized formulation was subjected to perform in vitro anti-fungal activity. Facility provided by Vasu Research Center, Baroda (Table 9).
| Culture | Blank | Control | Reference | Hydrogel–excluding Seed extract | Hydrogel |
|---|---|---|---|---|---|
| Candida albicans | NZ | 10 mm | 25 mm | 12 mm | 20 mm |
Table 9: Antimicrobial study data
Stability Study
Stability studies were carried out for statistically optimized formulation according to the International Council for Harmonization (ICH) Q1A (R2) guidelines. The samples were stored in closed high density polyethylene (HDPE) bottle which was maintained at room temperature 27° ± 2°/RH 65 ± 5% and accelerated conditions 40° ± 2°/RH 75 ± 5% for 45 days. The hydrogel formulation was withdrawn after a period of 30 days and 45 days and evaluated for physical appearance, pH, viscosity and drug content [17] (Table 10).
| Evaluation parameters | Initial | 30 days | 45 days | ||
|---|---|---|---|---|---|
| RT | 40°/75% RH | RT | 40°/75% RH | ||
| pH | 6.87 | 6.87 | 6.85 | 6.87 | 6.85 |
| Viscosity (cp) | 30130 | 30129 | 30127 | 30126 | 30125 |
| Drug content (%) | 98.75% | 98.73% | 98. 70% | 98.70% | 98. 65% |
Table 10: Stability study data of optimized formulation
Results and Discussion
QTPP serves as a summary of quality attributes of a drug product that need to be achieved to ensure its safety and efficacy. The QTPP for Herbal formulation consist of information regarding dosage form, dosage design and route of administration, dosage strength, and drug product quality attributes, and based on prior scientific knowledge and data an Initial Risk Assessment of drug substance (Herbal Extracts) and formulation variables (Excipients) was carried out to meet the Quality Target Product Profile (QTPP). Concentration of Carbapol 934p (%) (X1) and concentration of Aloevera gel extract (%) (X2) were considered as the major formulation parameters for design of experimentation. A 32 full factorial design was employed for development and optimization of Hydrogel. The experimental runs, with independent variables and measured responses for the developed hydrogel formulation are shown in (Table 2).
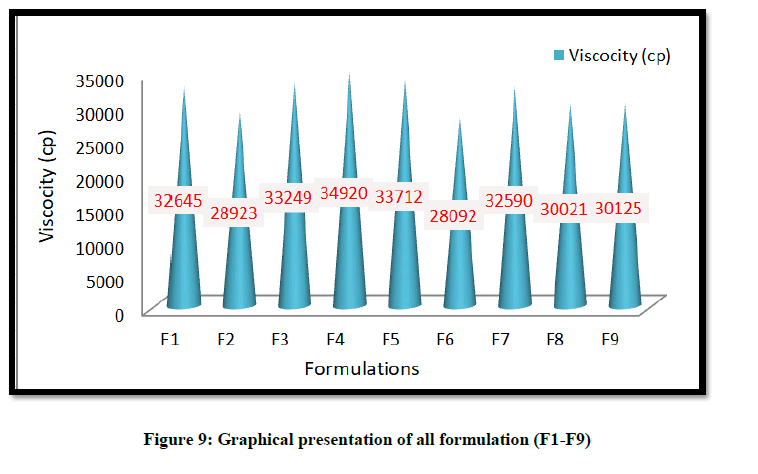
The all prepared formulations were inspected visually for their color, homogeneity, and grittiness. It observed that all the formulations were showed good homogeneity with no any grittiness and brown in color. The formulation batches F1-F9 were subjected for physical characterization, pH, spreadability, viscosity, drug release and drug content.
The pH of the hydrogel was found to be within 6.55-6.99. The viscosity of hydrogel prepared was in the range of 28092-34920 cp. The drug release (%CADD/cm2) of the hydrogel was found to be 50.12–62.53% and Coixol content was within the range of 97.12%-98.86%.
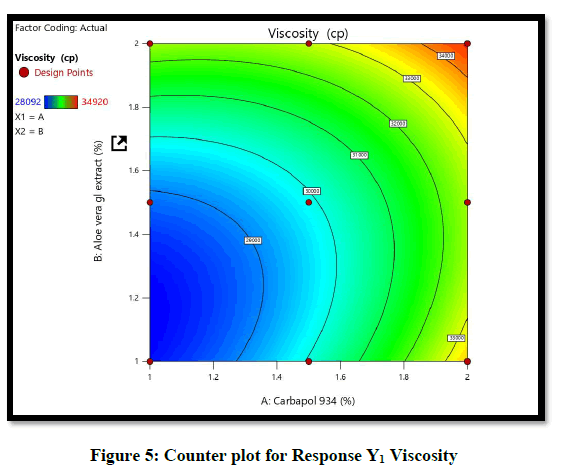
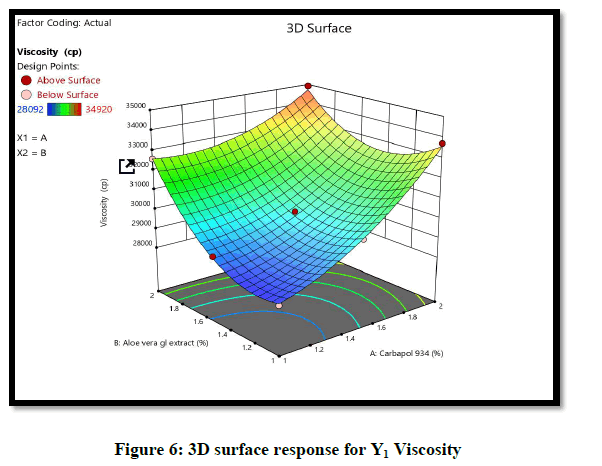
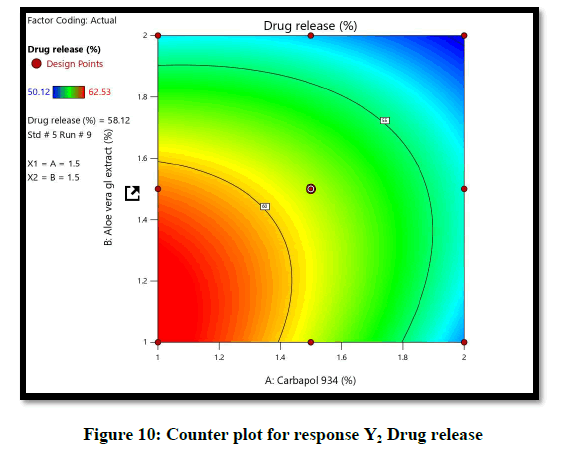
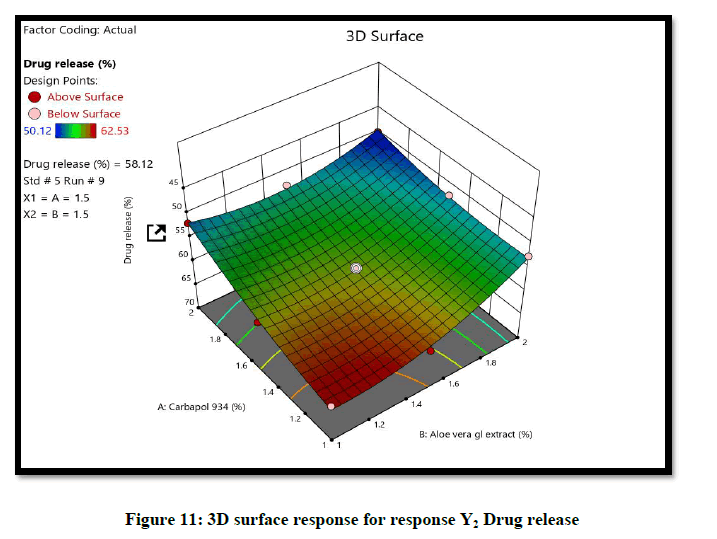
Statistical optimization of Herbal hydrogel was performed by comparison of several statistical parameters, provided by Design-Expert® Software, Version 12. The values of the coefficients X1, X2, their interaction and quadratic-terms are related to the effect of these variables on the response. To demonstrate graphically the influence of each factor on responses of viscosity the Contour plots, 3D surface plot, residual verses predicted and normal verses residual were generated (Figures 6-10).
The ANOVA result of response Y1: Coefficient of determination R2=0.9932, adjusted R2=0.9819, Predicted R2=0.9316 and p-value=0.0091 (p<0.05) and for response Y2: Coefficient of determination R2=0.9813, adjusted R2=0.9501, Predicted R2=07823 and p-value=0.00085 (p<0.05) (Tables 6 and 7). From the counter plot presentation, formulation no. 9–was within this appropriate area having viscosity 30125 cp. Based on 3D surface plot concentration of Aloevera extract was increased the pH of hydrogel also increase, so to maintain pH requirement of Triethanolamine was more and increased viscosity. To increase concentration of Aloevera gel extract in formulation decrease pH and to maintain pH need to add triethanolamine. So, it increased the viscosity of hydrogel. For responses of drug release the Contour plots, 3D surface plot, residual verses predicted and normal verses residual were generated (Figures 11-14).
From the graphical presentation formulation 9 was given adequate drug release which show in effective area of counter plot. The normal probability plot for both responses, the residuals is normally distributed.
From the Design Space, formulation batch F9 falls under the region of successful operating ranges. Hence formulation F9 (Carbapol 934p–1.5% and Aloevera gel extract-1.5%) fulfills the criteria of QTPP and CQA for hydrogel formulation. Therefore F9 formulation was selected as optimized formulation. The optimized formulation as evaluated for physical appearance, spreadability, viscosity, drug release and drug content (n=3) (Table 8).
In vitro antifungal activity performed by cylindrical plate method. Candida albicans (ATCC 10231) was used for antifungal activity. Sabroud dextrose agar with Chloramphenicol media was used to prepared agar plate. Four Derm Cream was used as a reference sample. The formulation showed antifungal activity having zone inhibition 20 mm (Figures 15 and 16, Table 9).
The results obtained demonstrated that Hydrogel formulation has good anti-fungal activity. The present research work has demonstrated the successful implementation of QbD approach for the development of hydrogel formulation. The desired QTPP and CQA were predefined in order to obtain the final product with desired quality. Further it can also be concluded that formulation prepared within the design space will be able to accomplish CQAs in the drug product which further results into the product with desired QTPP
Acknowledgment
The authors are thankful to the Indukaka Ipcowala College of Pharmacy, New V. V. Nagar, Vasu Research Center, Baroda, and Arogya Jyoti Pharma, New V.V. Nagar, Sardar Patel College of Pharmacy, Bakrol and authors also thanks to Dr. Bhavna Patel for their continues support and encouragement.
Conflict of Interest
The authors report no conflicts of interest.
References
- Kesarwani K, Gupta R. Asian Pac J Trop Biomed. 2013;3:253-66.
- Kamboj VP. Curr Sci. 2000;78:35-9.
- Patel G, Patel BA, Shah S, et al. J Pharmacogn phytochem. 2017;9:248-252.
- Chhabra D, Gupta RK. J Pharmacogn Phytochem. 2015; 4:291-98.
- Das S, Akhter R, Khandaker S, et al. J Pharmacogn Phytochem. 2017;5:360-64.
- Winfield AJ, Richards RM. 2004.
- Poapt B. Mohite, Sonali S, et al. Int. J Adv Pharm. 2017;6(3):79-85.
- Wing-Fu Lai, Andrey L. Rogach, et al. ACS Appl Mater. 2017;9:11309-1132.
[Cross Ref] [Google Schlor][PubMed]
- Guideline IH. ICH Topic. 1998; 6.
- Gaonkar VM, Mannur VS, Hullatti KK, et al. Indian J Pharm Sci. 2020; 82(4):640-9.
- Nagaria RK, Puranik SB. Int J Pharm Pharm Sci. 2019;14:130-151.
- ICH Q9.ICH.2005: 1-23.
- Pavia DL, Lampman GM, Kriz GS, et al. 2022.
- Aiyalu RK, Govindarjan AK, Ramasamy AK. Braz J Pharm Sci. 2016;52:493-507.
- Dev SK, Choudhury PK, Shrivastav R, et al. Biomed Pharmacother. 2019;111:555-67.
- Dombe S, Disale A, Pandhare R, et al. World J Pharm Pharm Sci. 2018;30:960-77.
- ICH Q1A (R2). ICH.2003